- MIC –
-
Minimal inhibitory concentration
- MBC –
-
Minimal bactericidal concentration
- WHO –
-
World Health Organization
- EO –
-
Essential oil
- FEMA –
-
Flavor and Extract Manufacturers Association
- FDA –
-
Food and Drug Administration
Abbreviations
1. INTRODUCTION
Dental caries, as a bacterially derived disease, is one of the most common public health problems (WHO). It is caused by a well-organized bacterial structure known as biofilm, in which the cariogenic bacteria Streptococcus mutans have been identified as the primary pathogenic agent (Kolenbrander et al., 2010). In addition, the initiation of dental biofilm formation, provided by the colonization of oral pathogens, is primarily attributed to Streptococcus genus bacteria (Sbordone and Bortolaia, 2003). It is common knowledge that complete elimination of biofilm through standard dental care techniques (e.g., brushing and flossing) is not often possible. Therefore, additional oral hygiene products are required, being used in the form of mouthwashes or oral gels. These products contain active antibacterial components, and among them, compounds based on chlorhexidine are the most widely used due to their proven effectiveness (Karamani et al., 2022; Pérez-Nicolás et al., 2023). Even though chlorhexidine-based formulations are undisputedly effective, some side effects may occur including changes in taste, numbness or discomfort in the tongue and lips, as well as dry mouth and teeth discolouration (Poppolo Deus and Ouanounou, 2022). Within dentistry, the efficacy of herbal extracts and essential oils (EOs) in preventing plaque and maintaining dental hygiene has been well-documented. These natural compounds possess promising activity against various oral infections and oral inflammation, used as both preventive measures and treatment options (Muresan et al., 2023).
EOs are secondary metabolites produced by different organs of aromatic plants. They are complex mixtures of terpenes and aromatic and aliphatic compounds of low molecular weight (Bakkali et al., 2008; Marinković et al., 2022). Numerous authors have reported their antioxidant, anti-inflammatory, antiviral, cytotoxic but also antimicrobial activity (Bakkali et al., 2008; Dorman and Deans, 2000). In the last few decades inhibitory potential of EOs against bacteria was in focus and their activity against oral ones was confirmed many times (Freires et al., 2015). Among them EOs rich in monoterpene carvacrol, such as Zataria multiflora and Origanum vulgare oils proved their efficiency against Streptococcus spp. (Aires et al., 2020; Yazdanian et al., 2022).
Previous studies demonstrated carvacrol’s antibacterial activity against S. mutans (Fernández-Babiano et al., 2022; Hoş et al., 2023; Hwang et al., 2004; Mathela et al., 2010; Miladi et al., 2017; Park et al., 2003; 2012; Wang et al., 2016; Zhang et al., 2023). However, results about its activity against S. sanguinis and S. mitis are still scarce (Fernández-Babiano et al., 2022; Hoş et al., 2023). Moreover, up to our knowledge, there is no information about carvacrol antibacterial potential against S. gordonii. Concerning antibiofilm activity, carvacrol was suggested as the one with antibiofilm potential against oral isolates including S. mitis, S. sanguinis, S. mutans, and S. gordonii (Maquera Huacho et al., 2019). For that reason in this study we monitored both, the antibacterial activity and antibiofilm activity of carvacrol against all four isolates belonging to Streptoococcus species.
2. MATERIAL AND METHODS
2.1. Bacterial strains and cultivation
Clinical isolates tested in the study, originated from patients at the Department of Pediatric and Preventive Dentistry, School of Dental Medicine, University of Belgrade, Serbia. Bacterial collecting and identification were undertaken according to the Declaration of Helsinki and approved by the Ethical Committee No 36/7. The bacteria cultivation was carried out by streaking frozen stock cultures onto Mueller Hinton Agar plates (MHA, Torlak, Belgrade, Serbia) followed by 48 h incubation at 37 ◦C. Individual colonies were grown overnight in Tryptic Soy Broth (TSB, Oxoid, Basingstoke, Hampshire, United Kingdom), at 37 ◦C. The optical density of bacterial suspensions was adjusted spectrophotometrically at 600 nm to achieve a value of 0.2 corresponding to bacterial inoculum of 1 × 108 CFU mL−1. All microbial procedures were carried out in a sterile environment.
2.2. The assessment of the antibacterial potential of carvacrol
Antibacterial potential of the monoterpene carvacrol was examined by the use of the broth-microdilution assay. Minimal inhibitory concentrations (MICs) as well as minimal bactericidal concentrations (MBCs) were determined according to the method previously outlined by (Vasilijević et al., 2019). Carvacrol was prepared by dissolving it in Tween 80 (2:3 v/v ratio) and subsequently serially diluted two-fold in TSB medium across the columns of 96-well microtiter plates. Its concentration range was 0.61 – 78.08 mg mL−1. Thereafter, an inoculum containing 2*104 CFU/well (105 CFU mL-1) of bacteria was added to each well. Resazurin was used as a growth indicator, and it was introduced at a final concentration per well of 0.0675 mg mL−1. In the assay, the negative control was the solvent Tween 80, while a positive control was the most commonly used antibiotic in dentistry (amoxicillin). Minimal bactericidal concentrations were determined by plating from the wells with no noticeable growth onto Mueller Hinton Agar plates. The experiment was performed in triplicate and repeated two times.
2.3. The assessment of the antibiofilm potential of carvacrol
The assessment was provided using crystal violet assay on 96-well flat-bottom plates (Stepanović et al., 2000) in order to investigate the carvacrol activity in the prevention of the biofilm formation as well as against already formed biofilm.
2.3.1. Prevention of biofilm formation
The impact of antibacterial agents on biofilm formation was determined according to previously described in Marinkovic et al. (2021). (2021). The bacterial cell suspensions (105 CFU/well) of four oral Streptococcus spp. isolates have been incubated simultaneously with the carvacrol (tested concentrations 2.44 – 19.52 mg mL-1, selected in accordance with the results of microdilution assay), at 37 ◦C, for 48 hours, in 96-well microtiter plates. Tryptic soy broth enriched with 0.5 % glucose has been used as a growth medium. After the 48 h required for the biofilm formation, the medium was aspirated and planktonic cells have been removed by twice washing with sterile saline. The cells have been air-dried and then the biofilm has been stained with 0.1 % crystal violet (Bio-Merieux, France). After 15 minutes, the wells have been washed, while the remaining stain, bounded to biofilm, was suspended in 96 % ethanol. The absorbance was read at 570 nm. The percentage of the inhibition of biofilm formation was calculated according to the Equation (1): (1)
AC and AT are absorbances at 570 nm of control and treatment, respectively.
2.3.2. Disruption of the pre-formed biofilm
In order to provide biofilm formation, inoculums of 2 × 105 CFU/well of each strain were added into polystyrene microtiter plates and incubated 48 h at 37 ◦C in order to allow biofilm formation. After that biofilms were exposed to the carvacrol in the same concentration range as previously mentioned. The disruption of biofilms was spectrophotometrically quantified at 570 nm, as described in previous section.
In both assays Tween 80 was used as negative control. Concerning its possible impact on biofilm forming prevention and biofilm disruption, its activity was taken into account in expressing carvacrol activity.
2.4. Statistical analysis
Results are presented as means ± standard deviation (SD). Group comparisons were performed using the One-Way Analysis of Variance test. All data were analysed using SPSS 20.0 (IBM Corporation) statistical software. All p values less than 0.05 were considered significant.
3. RESULTS
The inhibitory potential of carvacrol ( Table 1 ) was the same against S. mitis, S. gordonii, and S. mutans (MIC=1.22 mg mL-1), and slightly lower against S. sanguinis (MIC=2.44 mg mL-1). On the other hand, bactericidal effect differed among four isolates where S. gordonii (MBC=1.22 mg mL-1) was the most sensitive to its activity, while it was just an opposite in case of S. mutans (MBC=19.52 mg mL-1).
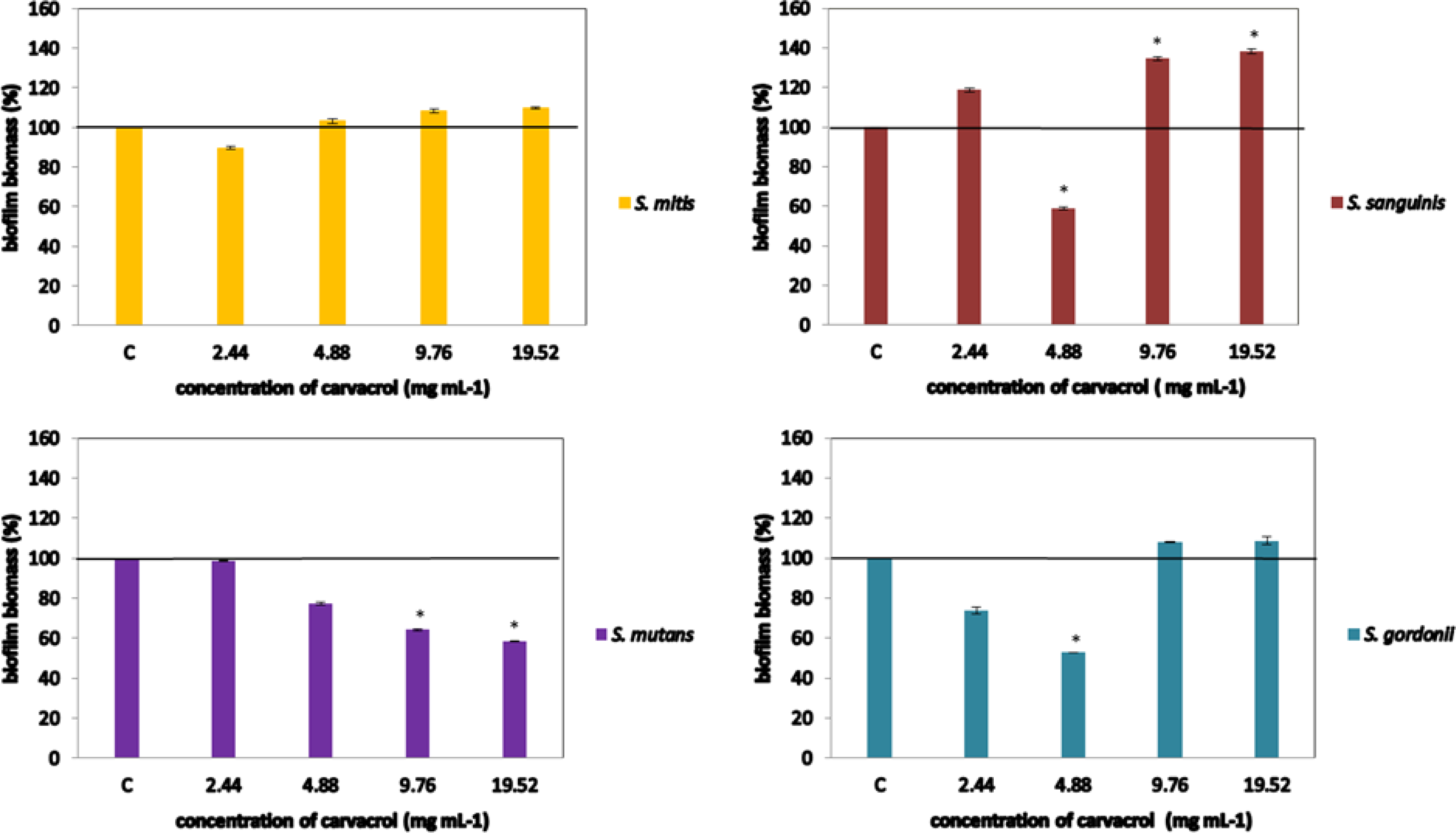
Concerning carvacrol antibiofilm potential, concentration of 4.88 mg mL-1 successfully inhibited its formation (41% and 47% for the S. sanguinis and S. gordonii, respectively). However, carvacrol effect on S. sanguinis biofilm formation was antagonistic: although inhibitory potential was detected for the conc. 4.88, higher concentrations significantly stimulate biofilm establishment. Inhibition of the S. mutans biofilm formation was successful when higher concentrations were tested (≥9.76 mg mL-1), while none of the tested concentrations had an impact on S. mitis biofilm Figure 1.
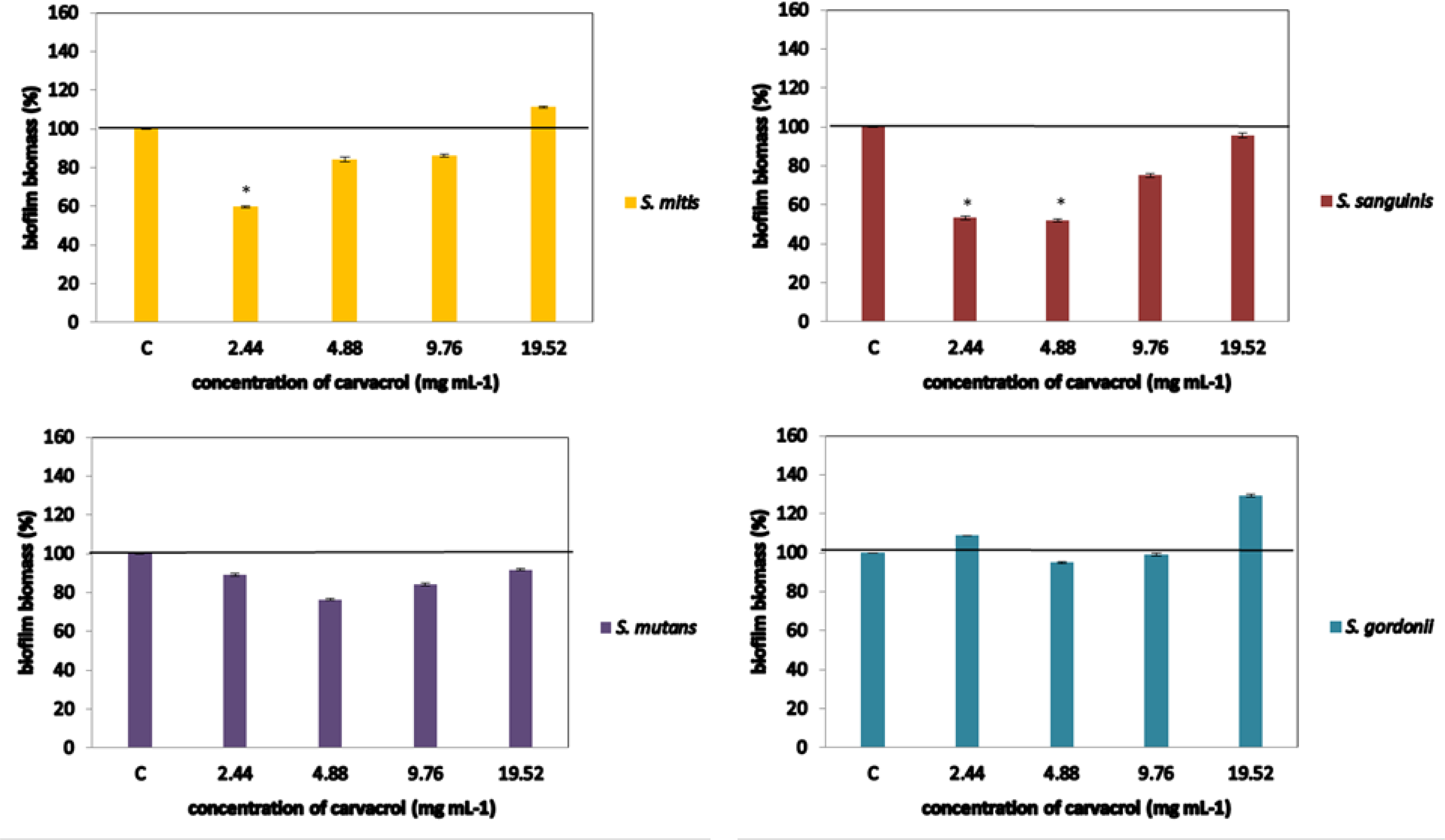
In case of the biofilm disruption, carvacrol was effective against S. mitis and S. sanguinis, inducing decrease of biofilm biomass for 40% and 46%, respectively, both at concentration 2.44 mg mL-1. In addition, the concentration 4.88 mg mL-1 reduced biofilm of S. sanguinis (48%). However, carvacrol could not affect already composed biofilm of S. mutans and S. gordonii regardless tested concentration Figure 2.
4. DISCUSSION
In this study, antibacterial and antibiofilm properties of the EO monoterpene carvacrol have been investigated. The selection of this monoterpene was provided in accordance to following: (1) some preparations commonly used in oral hygiene maintenance, such as Listerine, contain similar EO constituents (Marinković et al., 2023), and (2) carvacrol is generally recognized-as-safe (GRAS category) by the Flavor and Extract Manufacturers Association (FEMA) and the Food and Drug Administration (Magi et al., 2015). Furthermore, up-to date investigations of the antibacterial potential of EOs, containing carvacrol along with the studies exploring carvacrol solely, suggested its promising antibacterial and antibiofilm activity (Fernández-Babiano et al., 2022; Hoş et al., 2023; Hwang et al., 2004; Mathela et al., 2010; Miladi et al., 2017; Park et al., 2003; 2012; Wang et al., 2016; Zhang et al., 2023).
In the present study, antibacterial and antibiofilm potential of carvacrol was screened, with the initial hypothesis that, if the Streptococcus spp. biofilm formation could be prevented or already formed biofilm removed, then the use of carvacrol may reduce the incidence of tooth decays. To evaluate the antibacterial potential of carvacrol against the bacteria relevant to caries, the study focused on S. mitis, S. sanguinis, S. gordonii and S.mutans. S. mutans is broadly recognized as pathogenic and cariogenic bacteria. Other selected bacteria, i.e. S. mitis, S. sanguinis and S. gordonii are considered as commensals or bacteria with dual, beneficial, or detrimental roles in the host homeostasis (do Rosário Palma et al., 2018). However, they additionally showed some cariogenic potential that has been related to their capability to decrease oral pH, and consequently contribute to caries development (Banas et al., 2016).
Moreover, these species are also recognized as pioneers in biofilm onset (Kolenbrander et al., 2010). Taking into account aforementioned these bacteria could be considered as an adequate model for testing antibacterial/antibiofilm activity of test substances intended to be used in oral hygiene maintenance.
A pre-screening of antibacterial properties of carvacrol was performed using a microdilution assay and it revealed that carvacrol possess some antibacterial potential (MIC 1.22–2.44 mg mL-1). Slight differences in sensitivity among strains were observed, however more in the bactericidal effect in comparison to inhibitory one. Literature review showed that activity of carvacrol against S. mutans was explored and MIC ranged between 16 μg mL-1 and 2mg mL-1 Fernández-Babiano et al. (2022); Hoş et al. (2023); Hwang et al. (2004); Mathela et al. (2010); Miladi et al. (2017); Park et al. (2003; 2012); Wang et al. (2016); Zhang et al. (2023) which was in line with the present results. The only available information about the activity of carvacrol against S. sanguinis and S. mitis responded to MIC=93.4 μg mL-1 (Fernández-Babiano et al., 2022) and 1.90 mg mL-1 (Hoş et al., 2023), respectively, and the latter one is in line with our results. The activity of carvacrol against S. gordonii was for the first time monitored in this study. Possible explanation of the distinction in strain response compared to present study could be attributed to the genotypic and phenolic variances among the tested strain used in different studies (Marinkovic et al., 2021).
The screening of the prevention of biofilm biomass formation, revealed that carvacrol can prevent biofilm formulation of the S. sanguinis, S. gordonii and S. mutans, while the disruption was also occurred in case of biofilms of S. mitis and S. sanguinis. This is in agreement with the studiy presented by Maquera Huacho et al. (2019) that demonstrated the potential of carvacrol to prevent as well as to disrupt mature biofilms of aforementioned isolates (Maquera Huacho et al., 2019).
5. CONCLUSION
The evaluation of the antibacterial properties confirms that carvacrol has substantial antibacterial potential towards Streptococcus mitis, Streptococcus mutans, Streptococcus gordonii, and Streptococcus sanguinis. Carvacrol proved to be capable of preventing formation of the S. sanguinis, S. gordonii, and S. mutans biofilms. Furthermore, it also successfully disrupted biofilms of S. mitis and S. sanguinis. In light of the premise that prevention of Streptococcus species biofilm formation or removal of their pre-formed biofilms could affect dental caries prevalence, carvacrol may be a suitable candidate for incorporation into oral hygiene products.