1. INTRODUCTION
Vaccinium myrtillus L. (bilberry) represents a perennial, wild, and small deciduous shrub growing in the mountains and forests of Europe and belongs to the family of Ericaceae. The plant possesses significant economic importance due to the use of its fruit and leaf in numerous food, functional food, pharmaceutical, cosmetic, and health-care products (Vrancheva et al., 2021). Bilberry contains anthocyanins, phenolic acids, fatty acids, stilbenes, iridoid glycosides, dietary fibers, vitamins, and minerals (Jensen et al., 2002; Riihinen et al., 2008). The extracts prepared using bilberry leaves showed astringent, antioxidant, antibacterial, anti-inflammatory, hypolipidemic, and hypoglycemic effects (Jensen et al., 2002; Riihinen et al., 2008; Vrancheva et al., 2021).
In the present study, a microwave-assisted procedure was employed for the extraction of polyphenols from V. myrtillus leaves, as an innovative method for extracting valuable components from various plant materials, due to its higher extraction and polyphenol yields, shorter extraction time, lower amount of extraction medium, higher selectivity, and better quality of plant extracts, compared to traditional technologies (Ballard et al., 2010; Jovanović et al., 2021; Zhang et al., 2011).
The aims of the study were the preparation of V. myrtillus leaf extracts using different extraction mediums and temperatures in the microwave reactor, and the chemical characterization of the extracts via determination of the total polyphenol content (TPC), as well as measurement of their radical scavenging potential.
2. MATERIALS AND METHODS
2.1. Plant material and reagents
V. myrtillus leaves were purchased from the Institute for Medicinal Plants Research ”Dr Josif Pančić”, Pančevo, Serbia. The plant material was then ground into a very fine powder using a non-metallic electric grinder (particle size of ~0.3 mm). The plant material was kept in zipper storage bags in a dry and dark place until future extraction.
Distilled water was purified through a Simplicity UV® water purification system (Merck Millipore, Merck KGaA, Germany). Ethanol was obtained from Fisher Science (UK). For chemical spectrophotometric assays and/or for investigations of antioxidant activity following chemicals were used: sodium carbonate (Fisher Science, UK), Folin-Ciocalteu reagent and gallic acid (Merck, Germany), potassium persulfate (Centrohem, Serbia), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) or ABTS, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid or Trolox, and 2,2-diphenyl-1-picrylhydrazyl or DPPH (Sigma-Aldrich, Germany).
2.2. Microwave-assisted extraction
MAE was carried out using a microwave reactor, Monowave 300 (Anton Paar, Austria). A defined amount of V. myrtillus leaves (0.66 g) was mixed with 20 mL of the extraction medium (50% or 96% ethanol), in a closed reactor vial, using a magnetic stirring bar at the speed of 600 rpm for 2 min. MAE was performed using three different temperatures (60, 100, and 160 ◦C).
All extracts were filtered through a cellulose filter paper (fine pore, 0.45 μm) and stored at 4 ◦C until further analyses.
2.3. Polyphenol content of the extracts
The TPC values were determined using the modified Folin-Ciocalteu procedure (Skotti et al., 2014). The extract (100 μL) was added to distilled water (5 mL) and mixed with Folin-Ciocalteu reagent (500 μL). Subsequently, a 20% sodium carbonate solution (1.5 mL) was added, and the volume was made up to 10 mL with distilled water. The mixtures were left in the dark at room temperature for 100 min. The absorbance was measured at 765 nm against a blank (all reagents except the extract). Gallic acid was used as a standard for the calibration curve. The TPC was expressed as milligrams of gallic acid equivalents per g of plant material (mg GAE/g).
2.4. Antioxidant potential of the extracts
2.4.1. ABTS assay
The ABTS assay is based on the reduction of ABTS•+free radicals by antioxidant compounds from the sample (Re et al., 1999). A mixture of ABTS solution (5 mL) and potassium persulfate solution (88 μL) was left to react for 16 h in a refrigerator. The ABTS•+working solution was diluted using ethanol (an absorbance of ~0.700 at 734 nm). ABTS•+solution (2 mL) was mixed with diluted liquid extract (1:9, 20 μL). After 6 min of incubation, the absorbance was read and the ABTS radical scavenging activity of the extract was calculated using the following Equation 1: (1) where A0was the absorbance of ABTS•+solution, whereas Axwas the absorbance of ABTS•+solution and the extract. Trolox was used as a standard for the calibration curve. The scavenging capacity was expressed as μmol Trolox equivalents per g of plant material (μmol TE/g).
2.4.2 DPPH assay
The antioxidant activity of the samples was determined via hydrogen donating or radical scavenging ability using the stable DPPH•radicals (Batinić et al., 2022). Various concentrations of liquid extract (200 μL) were mixed with 2.8 mL of ethanol DPPH•radical solution (an absorbance of ~0.800 at 517 nm). The absorbance was recorded after 20 min of incubation and the percentage of inhibition was calculated using the following Equation 2: (2) where A0was the absorbance of the control, and Axwas the absorbance of DPPH•solution and extract. The results were expressed as IC50(mg/mL) which represented the concentration of the extract required to scavenge 50% of DPPH•radicals.
All absorbance readings are performed using the UV Spectrophotometer UV-1800 (Shimadzu, Japan). Every spectrophotometric measurement was performed in triplicates.
2.5. Statistical analysis
The statistical analysis was performed by using the analysis of variance (one-way ANOVA) followed by Duncan’s post hoc test, within the statistical software STATISTICA 7.0. The differences were considered statistically significant at p<0.05, n=3.
3. RESULTS AND DISCUSSION
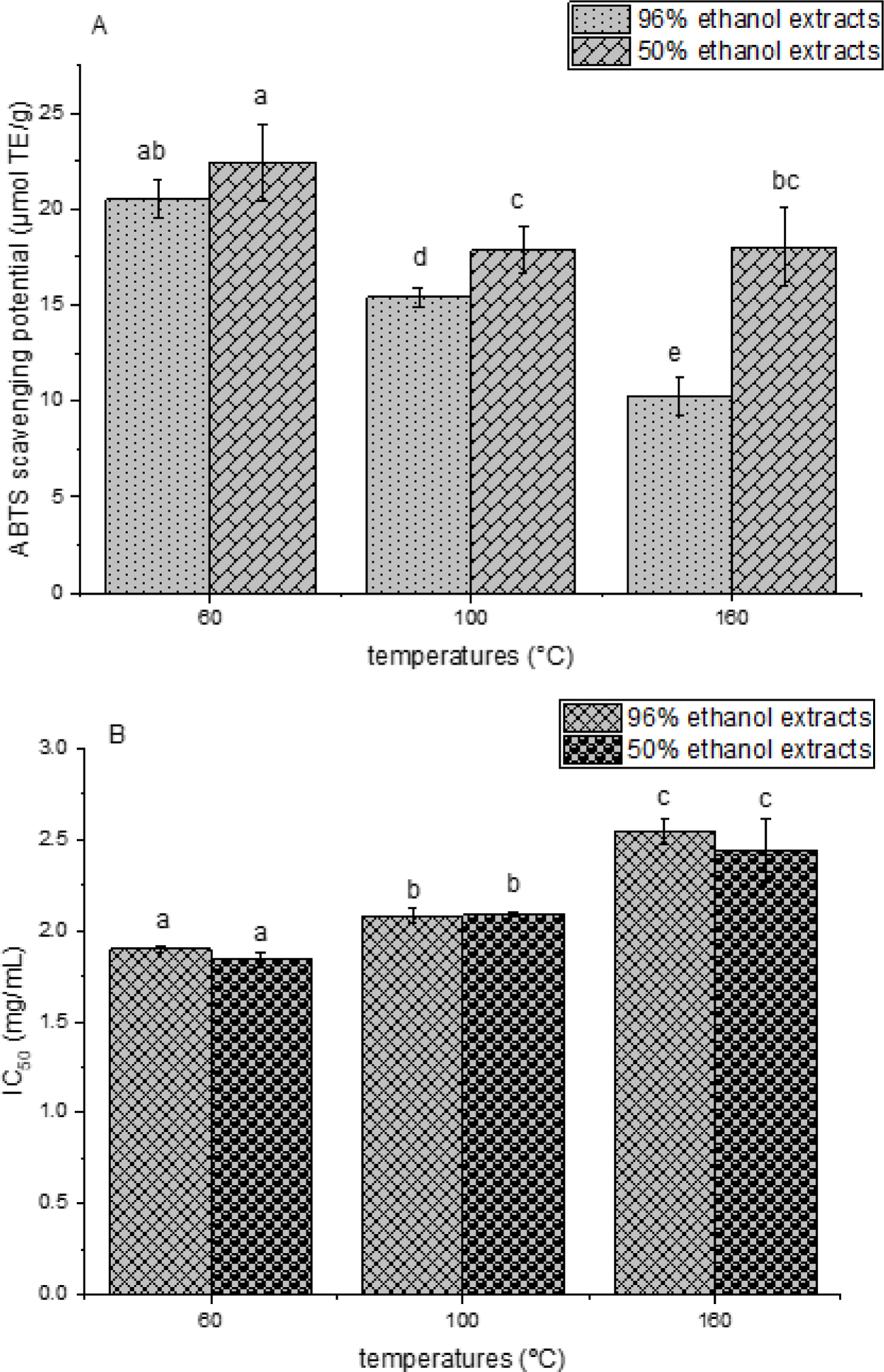
In the present study, microwave extraction from V. myrtillus leaves was performed by investigating factors of interest: solvent type and extraction temperature. The extraction was performed using a microwave reactor (a modern procedure that requires an expensive device but provides faster kinetic). The results of polyphenol yield are presented in Table 1, while the data of ABTS and DPPH radical scavenging potential are shown in Figures 1A and 1B.
| Solvent type | Extraction temperature (◦C) | TPC (mg GAE*/g) |
|---|---|---|
| 96% ethanol | 60 | 37.2±0.5d |
| 100 | 46.6±0.3c | |
| 160 | 55.1±0.5b | |
| 50% ethanol | 60 | 54.9±1.0b |
| 100 | 58.3±1.0a | |
| 160 | 58.0±1.5a |
As can be seen from Table 1, the polyphenol yield of the absolute ethanol V. myrtillus leaf extracts exponentially rose with the increase of the extraction temperature (from 37.2±0.5 mg gallic acid equivalents (GAE)/g of plant material at 60 ◦C to 55.1±0.5 mg GAE/g at 160 ◦C). The polyphenol concentration of the 50% ethanol extract prepared at 60 ◦C was significantly lower (54.9±1.0 mg GAE/g) compared to the samples prepared at 100 and 160 ◦C (58.3±1.0 and 58.0±1.5 mg GAE/g, respectively). The obtained data are in agreement with the literature, where the TPC of ethanol wild thyme extracts significantly increased with the increase of the temperature in the microwave reactor (Jovanović et al., 2022). Namely, in microwave-assisted extraction, there is a rapid delivery of energy to the extraction medium and plant material resulting in the efficient and homogeneous heating of the whole sample. Furthermore, water in the plant cells and vacuoles absorbs microwave energy causing interior superheating and degradation of plant cells and tissues, consequently resulting in the enhancement of the extraction and polyphenol yields (Ballard et al., 2010; Jovanović et al., 2022; Zhang et al., 2011). The temperature of microwave-assisted extraction should be investigated and optimized, as microwave irradiation can cause enzymatic degradation and oxidation of polyphenol compounds, particularly at higher temperatures (Wang and Weller, 2006). Regarding the comparison of the extracts prepared using different extraction solvents, it can be noticed that 50% ethanol extracts showed significantly higher values of the TPC at all levels than the extract with absolute ethanol. According to the literature, ethanol-water mixtures have been usually employed for the extraction of polyphenol compounds from different plant sources, because ethanol provides a decrease in the dielectric constant of the extraction medium enabling easier separation of the solvent molecules, while water allows the efficient wetting of plant solid (Costa et al., 2012; Jovanović et al., 2021; Pompeu et al., 2009). However, in 50% ethanol extracts, there was no statistically significant difference between the samples obtained at 100 and 160 ◦C which can be explained by the saturation of the extraction medium (Gao et al., 2007). Hence, steady state was achieved already at 100 ◦C in the case of 50% ethanol extracts due to better solubility of the polyphenolic compounds in ethanol-water surrounding, while in pure ethanol extract, the temperature in the microwave reactor had to be 160 ◦C for the highest polyphenol yield.
The ABTS and DPPH radical scavenging ability of ethanol V. myrtillus leaf extracts did not correlate with the polyphenol yield and significantly decreased with the increase of the extraction temperature in the case of both extraction mediums (Figure 1). It can be explained by the fact that other components, apart from polyphenols, can have an important role in the overall antioxidant capacity of the extracts (plant pigments, vitamins, free organic acids, sugars, etc.) (Petrovic et al., 2019; Özyürek et al., 2011). However, the mentioned compounds can be significantly degraded under higher temperatures. Additionally, flavonoids, as a subgroup of polyphenols, represent potent antioxidants (Carocho and Ferreira, 2013), as well as a higher sensitivity to high temperature, particularly during microwave-assisted extraction (Liazid et al., 2007). On the other hand, the Folin-Ciocalteu method is not a selective method and can quantify the content of proteins apart from the polyphenols (Sánchez-Rangel et al., 2013). However, plant proteins do not show significant radical scavenging potential that can explain the absence of the correlation between the TPC determined in the Folin-Cioacalteu assay and the antioxidant capacity of the extracts. Nevertheless, ABTS antioxidant activity was higher in the 50% ethanol samples in comparison to 96% ethanol parallels, as in the case of TPC. In the DPPH assay, there was no statistically significant difference between the 50 and 96% ethanol extracts. Probably, both solvents can extract the compounds responsible for the neutralization of free DPPH radicals.
4. CONCLUSION
The aim of the present study was the optimization of microwave-assisted extraction via varying the extraction solvent and temperature, measurement of polyphenol yield and ABTS and DPPH radical scavenging effects of bilberry leaf extracts. Polyphenol concentration of the extracts rose with the increase of the extraction temperature, while the highest polyphenol yield was achieved employing 50% ethanol, as an extraction medium compared to the absolute ethanol. Nevertheless, the ABTS and DPPH radical scavenging capacity of the extracts did not correlate with the polyphenol content at all levels. Namely, the antioxidant capacity decreased with the increase of the temperature in the microwave reactor, whereas 50% ethanol extracts showed higher ABTS radical scavenging capacity in comparison to 96% ethanol parallels. Therefore, the extraction temperature should be chosen depending on the future application of bilberry extracts, while due to higher polyphenol yield and anti-ABTS effect and reduced consumption of organic solvent, a 50% ethanol V. myrtillus extract was favored. The present research was an initial step in the preparation of bilberry extracts in microwave-assisted extraction which can be potentially implemented in food, pharmaceutical, and cosmetic formulations.