1. INTRODUCTION
In recent years, the use of dietary supplements, as a means of preventing various health complications, has seen a remarkable increase. A key challenge for the dietary supplement industry is to improve production capacity while adhering to the highest principles of protecting human health and the environment. In the number of dietary supplements herbal extracts are the primary bioactive constituents of the product. They are produced using different extraction techniques and solvents. A forward-looking approach in herbal extract production suggests replacing the conventional, often hazardous solvents used in the manufacturing processes, with environmentally friendly alternatives. The increased market demand for these kinds of supplements and extracts has led the scientific community to explore an expanded repertoire of solvents suitable for the precise isolation of the various bioactive constituents including polyphenolic compounds.
This endeavor includes the development of innovative extraction techniques specifically designed to achieve these goals. Among the solvents that have been favored in the past for the commercial production of extracts with a high concentration of phenolic compounds, water, and ethanol in varying concentrations are the most recommended. In addition to these established solutions, the potential of other solvents for the isolation of compounds, including polyphenols, is currently being explored. These include glycerol (Kowalska et al., 2021), methanol (Domínguez et al., 2020; Manizabayo et al., 2019), citric acid (Pliszka, 2017), acetone (Zhou et al., 2020), deep eutectic solutions (Perna et al., 2020; Vladić et al., 2023) and others. Apple cider vinegar (ACV) is obtained by the dual process of acetate and alcoholic fermentation of apples or concentrated apple juice, as explained by Joshi and Sharma (2009). ACV serves as a reservoir of bioactive compounds and vital nutrients, including polyphenols, organic acids, sugars, melanoidins and other components, as confirmed by Ho et al. (2017), Xia et al. (2020) and Budak (2021). The quality and sensory properties of ACV are closely related to its production method. As Chen et al. (2016) explained, the composition and concentrations of the ingredients of ACV depend on the selection of the raw materials, the production technology used and the specific processes. The various ingredients of ACV play a central role in the treatment and prevention of diseases due to their recognized anti-inflammatory and antibacterial properties, as stated by Hindi (2013). In addition, these ingredients have effects on weight management, obesity prevention, and regulation of blood lipid levels (Hadi et al., 2021). There are two main methods for the production of ACV: the traditional method and the industrial (commercial) method. The traditional method, although time-consuming, involves the sequential alcoholic and acetic acid fermentation of apple juice with natural yeasts and acetic acid bacteria (AAB). In modern times, ACV is mainly produced by submerged cultivation, similar to the production of wine vinegar (Joshi and Sharma, 2009).
Elderberry (Sambucus nigra L.), a small tree or wild deciduous shrub, has a remarkable range of medicinal properties. Extensive literature highlights its diverse therapeutic uses, including the treatment of respiratory and rheumatic conditions, as well as its use as a diuretic and laxative (Hawkins et al., 2019). Elderberry fruits are particularly rich in secondary metabolites consisting mainly of polyphenolic compounds, including flavonols, phenolic acids, proanthocyanidins, and anthocyanins, which are responsible for the characteristic purple color of the fruit. These compounds have been well described by Domínguez et al. (2020). The main anthocyanins identified in elderberries are derivatives of cyanidin, with cyanidin-3-glucoside and cyanidin-3-sambubioside predominating alongside traces of cyanidin-3-sambubioside-5-glucoside, cyanidin-3,5-diglucoside, cyanidin-3-rutinoside, pelargonidin-3-glucoside, pelargonidin-3-sambubioside and delphinidin-3-rutinoside. Elderberries also contain important flavonoids, namely quercetin, kaempferol, isoquercetin, and rutin, which contribute to their therapeutic efficacy, as shown in the study by Avula et al. (2022). In addition, elderberries contain chlorogenic acid, derivatives of caffeic acid, and traces of p-coumaric acid and ellagic acid (Terzić et al., 2022).
As already documented in the scientific literature, the quality of herbal extracts depends on several crucial factors, including the choice of extraction agent, the quality of the plant material under consideration, and the extraction methodology employed (Chemat et al., 2017; Sulejmanović et al., 2024). The ultrasound-assisted extraction (UAE) stands out as a superior alternative to conventional techniques that offer significant advantages in terms of energy efficiency, time efficiency, solvent consumption, and ease of subsequent solvent removal. The application of ultrasound in extraction processes is characterized by its simplicity, adaptability, feasibility on an industrial scale and lower investment requirements, especially compared to high-pressure techniques (Tiwari, 2015).
In view of these considerations, the main objective of this research project was to evaluate the suitability of ACV as an alternative solvent for the extraction of bioactive compounds from elderberry fruits using an advanced and environmentally friendly UAE method. Given the lack of scientific data in this field, a comparative analysis was performed between the extraction of elderberry fruit with ACV and ethanol, a conventional and widely used solvent. In the first phase, a solid-liquid extraction (SLE) was performed with ground elderberries using different concentrations of ethanol (30%, 50%, and 70% (v/v)) and ACV (seven different ACVs sourced from different manufacturers and produced according to different protocols). After identifying the most suitable extraction solvents, the influence of UAE parameters on the extraction of polyphenols from elderberries was investigated. The studies included a range of extraction durations from 120 to 360 s and investigated the effects of three different sonication amplitudes, ranging from 20% to 100%. The optimization of the process conditions for the production of elderberry fruit extracts was achieved by a comparative evaluation of the extraction yield. In this context, this study investigates the feasibility of using ACV as an alternative solvent for the isolation of phenolic compounds from elderberries. The underlying premise of this investigation was whether we can utilize the existing commercial liquid natural product, of the proven health benefits, to selectively isolate the bioactive compounds, such are phenolics, and thus increase the value of the resulting extracts. To evaluate the potential of ACV as a solvent for the extraction of polyphenols, a case study was conducted using elderberry fruit as a starting material for extraction and then subjected to a comprehensive analysis.
2. MATERIALS AND METHODS
2.1. Herbal material and chemicals
Commercially available dried berries of Sambucus nigra L. originating from the Institute “Dr. Josif Pančić” in Belgrade, Serbia formed the primary plant material for extraction in this study. Prior to extraction, these dried elderberries were mechanically crushed in a blender. The average particle size of the resulting material was determined using a sieve set (CISA Cedaceria Industrial, Spain). The milled material was subsequently stored in a controlled environment, i.e., in a dark and dry place at room temperature, to prepare it for subsequent analysis.
Various ACVs from different manufacturers were used for the study, including Aroma, Body Guard, Ekofarm, and Status. In addition, ethanol with a concentration of 96% was obtained from the manufacturer Centrochem, Šabac, Serbia. The chemical reagents Folin–Ciocalteu reagent and 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) were purchased from Sigma-Aldrich (Steinheim, Germany). All other chemicals used in this study were in accordance with analytical standards.
2.2. Conventional solid-liquid extraction
The initial phase of this study, SLE was used to investigate the range of solvents available for the extraction of phenolic compounds from elderberries. During the SLE procedure, 5 g of the plant material was subjected to the extraction with 50 mL (1:10) of various solvents. These solvents included aqueous ethanol solutions at concentrations of 30%, 50%, and 70% (v/v) ethanol and undiluted ACVs from different manufacturers, each prepared according to different protocols. BodyGuard’s ACVs, referred to as ACV 1, ACV 2, and ACV 3, and Aroma’s ACVs, referred to as ACV 5 and ACV 6, were obtained by traditional manufacturing processes. In contrast, the ACVs from Status and Ekofarm, designated ACV 4 and ACV 7 respectively, were produced using accelerated industrial processes. The extraction processes were carried out at a controlled temperature condition of 25 ◦C over a period of 24 h, without mechanical agitation. After the extraction process, the obtained extracts were filtered through filter paper under vacuum conditions. After filtration, the extracts were safely bottled and stored at 4 ◦C until they were subjected to subsequent analytical tests. The extraction yield (EY) was expressed as the mass of dry extract (g) per g of dry plant material, i.e., the percentage (%).
2.3. Ultrasound-assisted extraction
The UAEs were performed with different sonication amplitudes, namely 20%, 60% and 100%, over different time intervals of 120, 240, and 360 s. These extractions were performed with a specific drug-solvent ratio of 1:10, whereby 10 g of the plant material was mixed with 100 mL of the intended solvent. Based on the results of the SLE, water-diluted ethanol (30% ethanol, v/v) and ACV 4 were selected as optimal solvents for the UAE. Throughout the extraction process, changes in temperature and energy consumption were closely monitored. The UAE procedures were performed using an ultrasonic probe system from Hielscher Ultrasonic GmbH (Teltow, Germany), supplemented with magnetic stirrer to enable continuous mixing. After completion of the extraction phase, the resulting extracts were filtered through filter paper under vacuum conditions. Subsequently, these extracts were systematically collected in storage vessels and kept at a controlled temperature of 4 ◦C until the time of analysis.
2.4. Total phenolic, and anthocyanins contents
Total phenolic content (TPC) was determined by the Folin-Ciocalteu method as described by Singleton and Rossi (1965). Gallic acid was used to generate a standard curve. The absorbance of the samples was quantified at a wavelength of 750 nm using a Jenway 6300 spectrophotometer (Cambridge, UK). The TPC values were subsequently expressed in milligrams of gallic acid equivalent (GAE) per gram of dry extract (DE).
Quantification of total monomeric anthocyanin content (TAC) was performed using the pH differential method according to the protocol described by Lee et al. (2005). Potassium chloride (0.025M) at a pH of 1.0 and sodium acetate (0.4M) at a pH of 4.5 were used to prepare the required buffer solutions. The absorbance of the samples was measured at two different wavelengths, 520 nm and 700 nm. The TAC values were expressed in milligrams of cyanidin-3-glycoside (C3G) per liter of extract.
2.5. Antioxidant activity
The evaluation of radical scavenging activity in the samples was performed using the DPPH assay, a method previously described by Espin et al. in 2000. The absorbance at a specific wavelength, exactly 515 nm, was precisely quantified. The results were then expressed as radical scavenging capacity (RSC) in percent (%) and inhibitory concentration (IC50), which indicates the concentration at which 50% of the radicals are inhibited.
2.6. Statistical analysis
The analyses were performed carefully and according to strict scientific standards in triplicate. The resulting data were presented as mean values with the corresponding standard deviations (SD). This approach ensures the robustness and reliability of the results obtained. To determine meaningful differences between the results, we performed a one-way ANOVA analysis followed by a Tukey test. Significance was recognized when p < 0.05, n=3.
3. RESULTS AND DISCUSSION
3.1. Characterization of ACVs
The potential of ACV as an alternative solvent was explored by Mohammed et al. (2021). Their study involved the extraction of bioactive compounds from roselle, green tea, and clove with ACV to increase the antimicrobial activity of these extracts. The study yielded promising results suggesting that ACV, which is also rich in phenolic components, can contribute significantly to the bioactivity of the resulting plant extracts. According to scientific findings, the phenolic compounds in ACV mainly include gallic acid, catechin, epicatechin, chlorogenic acid and p-coumaric acid (Budak, 2021; Tripathi and Mazumder, 2020). To gain a deeper insight into the subsequent extraction process and to evaluate the role of ACV in shaping the properties and bioactivity of the final extract, the ACV was subjected to further characterization. This included the evaluation of dry weight (DW), TPC and antioxidant activity. At the same time, the pH values of the ACV were monitored and values between 2.66 and 3.65 were consistently observed. These pH values are consistent with the results already published (Budak et al., 2011). However, it is noteworthy that different pH ranges for ACV are reported in the literature such as 3.18-3.83 (Ousaaid et al., 2021; Ozturk et al., 2015).
Table 1 shows the differences in DW and TPC observed between the different ACVs, a variation that is probably due to the different production methods and raw materials used. ACV 5 had the highest DW at 20.73 mg/mL, while ACV 2 had the lowest DW at 7.69 mg/mL. The ACV 1-3 are vinegars produced using the same process and by the same manufacturer, but from a different batch. This observation underlines that despite identical production processes, the quality of the ACV differed and depended on the quality of the raw materials. Consequently, these differences in raw materials and processing had a recognizable influence on the properties of the final ACVs, as shown by the significant differences in DW value between the studied ACVs (Table 1). In the study by Karadag et al. (2020), traditional ACV production resulted in an ACV of 9.5% DW. A comparison of the results of Karadag et al. (2020) with the DW content of ACV 5 (20.73%), which was also produced by traditional methods, showed that the DW of ACV 5 was more than twice as high, although both were produced by the same method. Furthermore, the analysis revealed a significant difference in TPC in the ACV analyzed. The highest total TPC was detected in ACV 2 with 60.32 mg GAE/g DE, while ACV 7 had the lowest TPC with 2.64 mg GAE/g DE. In contrast to present study, Sengun et al. (2019) found TPC values of 1.02 and 0.988 mg GAE/mL for grape and apple cider vinegar, respectively, which is approximately 60 times lower than the current results for the ACVs studied.
| pH | Dry weight (mg/mL) | TPC (mg GAE/g DE) | IC50 (μg/mL) | |
|---|---|---|---|---|
| ACV 1 | 3.43 | 12.64 ± 0.68c | 29.54 ± 3.04c | 42.54 ± 0.83f |
| ACV 2 | 3.65 | 7.69 ± 0.65d | 60.32 ± 2.23a | 39.73 ± 0.74f |
| ACV 3 | 3.28 | 13.52 ± 1.03bc | 52.49 ± 1.48b | 82.62 ± 1.76e |
| ACV 4 | 3.1 | 16.17 ± 1.04b | 7.22 ± 0.45d | 226.16 ± 10.86c |
| ACV 5 | 3.1 | 20.73 ± 2.05a | 5.74 ± 0.40d | 251.69 ± 5.21b |
| ACV 6 | 3.1 | 15.71 ± 0.32b | 8.44 ± 1.36d | 160.86 ± 3.91d |
| ACV 7 | 2.66 | 20.63 ± 0.29a | 2.64 ± 0.64e | 931.76 ± 14.20a |
Unequal letters within a column indicate a significant difference between the samples with a significance level of p < 0.05, n=3. ACVs, apple cider vinegars; TPC, total phenolic content; IC50, inhibitory concentration at which 50% of the radicals are inhibited.
3.2. Characterization of extracts obtained by SLE
The SLE serves as a valuable benchmark for evaluating the feasibility of new solvents and the efficacy of alternative techniques (Naviglio et al., 2019; Živković et al., 2022). This approach is characterized by the fact that a significant volume of solvent is required and it takes a longer time for solvent to permeate the raw material and facilitate the extraction of the target compounds. In this study, SLE was strategically used as a tool to identify the most suitable solvent to optimize the extraction of berry constituents and obtain high-quality extracts in the further advanced extraction process. Besides, to get an assumption on the scaled process feasibility, the subtletyof the separation process, where the obtained extract was separated from the residues, was also observed in the selection of a suitable solvent. The qualitative evaluation of the extracts obtained in the SLE, considering parameters such as EY, TPC, and antioxidant activity, led to the selection of the two most promising solvents for further studies within the UAE.
In the experiments where ethanol was used for the extraction of elderberry fruit, the EY varied between 18.83% and 37.07%. The highest EY (37.07%) was obtained with a 30% ethanolsolution. Accordingly, the TPC values also reflect this trend, with the 30% ethanol solution proving to be the most effective. In the same extract, produced using 30% ethanol, aquantified TPC of 82.89 mg GAE/g DE was obtained, while the lowest concentration was observed in the extract obtained with 70% ethanol as extraction solvent (Table 2). Duymuş et al. (2014) reported that the TPC values, ranging from 49.17 to 89.74 mg GAE/g DE, are to be expected in the dried elderberry fruit extracts where the extraction was carried out through SLE using various solvents, including water, 70% ethanol, and methanol. It is worth mentioning that our previous study also confirmed the suitability of the 30% ethanol solution for the extraction of phenolic compounds from elderberry pomace (Mutavski et al., 2022).
| Solvent | EY (%) | TPC (mg GAE/g DE) | IC50 (μg/mL) |
|---|---|---|---|
| 30% ethanol | 37.07 ±1.50f | 82.89 ± 4.23a | 32.54 ± 1.71c |
| 50% ethanol | 31.11 ± 0.72g | 76.60 ± 4.52ab | 34.04 ± 2.55c |
| 70% ethanol | 18.83 ± 0.68h | 48.18 ± 4.40g | 56.42 ± 3.12b |
| ACV 1 | 53.48 ± 1.96ab | 66.36 ± 2.70cd | 69.82 ± 4.95a |
| ACV 2 | 42.56 ± 1.57e | 75.39 ± 4.75b | 51.61 ± 3.62b |
| ACV 3 | 44.93 ± 1.13de | 67.72 ± 1.37c | 53.64 ± 4.95b |
| ACV 4 | 51.69 ± 1.40bc | 61.42 ± 1.96cde | 55.71 ± 3.91b |
| ACV 5 | 57.37 ± 2.32a | 55.05 ± 3.40ef | 55.42 ± 5.42b |
| ACV 6 | 49.54 ± 1.86bc | 60.27 ± 2.82def | 54.75 ± 4.95b |
| ACV 7 | 48.18 ± 1.55cd | 53.83 ± 1.48fg | 60.72 ± 3.42ab |
Unequal letters within a column indicate a significant difference between the samples with a significance level of p < 0.05, n=3. EY, extraction yield; SLE, solid-liquid extraction; TPC, total phenolic content; ACV, apple cider vinegar.
Also, the elderberry fruit ethanolic extract obtained with 30% ethanol as solvent showed the highest antioxidant activity (IC50 value of 32.54 μg/mL). This result is consistent with the highest TPC observed in the same extract. Oniszczuk et al. (2019) determined IC50 values of 3.17, 3.68, and 3.79 in elderberry extracts obtained during 15, 30, and 60 min of UAE with 80% ethanol, respectively. Interestingly, in this study an increase in the extraction time led to a decrease in the antioxidant activity of the extracts, which could be due to the time degradation of the bioactive compounds under the set process conditions. Some studies have reported the opposite results (Domínguez et al., 2020). By varying the concentrations of ethanol in the extraction solvent, they found out that 50% ethanol solution was the most effective for extracting the phenolic compounds from the elderberries. These discrepancies may be due to different experimental conditions and methods used in their study compared to the present investigation.
The EYs of the extracts obtained using ACVs as extraction solvents were between 42.56% and 57.37%. Application of ACV 2 resulted in the lowest yield, while the highest was obtained using ACV 5. However, the extract obtained using ACV 2 as solvent resulted in the highest TPC with 75.39 mg GAE/g DE, while the lowest TPC value was measured in the extract obtained by ACV 7. It is important to emphasize that the phenolic compounds of ACV extracts exceed that of pure ACVs. According to the obtained results this was especially case in the extracts obtained by application of ACVs 4 and 5 (ACVs obtained from traditional production) and ACVs 6 and 7 (obtained from industrial production), where TPC was around 10 times higher in the extracts than in the pure ACVs. This indicates that these ACVs are efficient extraction solvents for elderberries‘ phenolics. The increase was also noticed in the application of ACVs 1, 2, and 3, but it was not so conspicuous. The antioxidant activity of the elderberry ACV extracts ranged from IC50 51.61 μg/mL to 69.82 μg/mL. The antioxidant activity generally was also multiplied in the case of ACVs extracts in comparison to the activities of pure ACVs (except in the case of ACVs 1-3), what is also confirming the assumption that some ACVs are the efficient systems for the elderberries‘ phenolics isolation.
In the ACV elderberry extracts production, problems occur during the filtration of the extracts obtained by the ACV 1-3, such as the clogging of the pores of the filter paper and the inability to separate the liquid medium. Therefore, they were not considered as alternative solvents for further application in the UAE. It can be assumed that due to this, their application on the industrial scale would likely be associated with significant technical complications. Therefore, ACV 4, with highest TPC value of 61.42 mg GAE/g DE, was identified as an optimal solvent for producing elderberry fruit extract with an alternative solventand for further exploration of its potential in the context of UAE (Table 3).
| 30% ethanol | ACV 4 | ||||||
|---|---|---|---|---|---|---|---|
| A | t (s) | EY (%) | TPC (mg GAE/g DE) | TAC (mg C3G/L) | EY (%) | TPC (mg GAE/g DE) | TAC (mg C3G/L) |
| 120 | 21.64 ± 0.56f | 100.63 ± 4.28ab | 12.21 ± 0.37f | 39.55 ± 0.88e | 65.71 ± 2.26d | 7.05 ± 0.22c | |
| 20% | 240 | 24.12 ± 0.79ef | 98.36 ± 2.87abc | 15.17 ± 0.36de | 39.94 ± 1.73de | 69.33 ± 2.86bcd | 7.21 ± 0.29bc |
| 360 | 27.70 ± 1.20e | 89.16 ± 3.40abc | 16.84 ± 0.47d | 43.78 ± 1.64cde | 66.54 ± 2.80cd | 7.82 ± 0.36abc | |
| 120 | 36.18 ± 1.04d | 85.49 ± 9.87c | 30.37 ± 0.63a | 44.58 ± 2.11be | 62.83 ± 5.29d | 7.88 ± 0.20abc | |
| 60% | 240 | 37.06 ± 0.93d | 95.17 ± 15.46abc | 11.69 ± 0.35f | 43.62 ± 0.98cde | 67.05 ± 3.42cd | 7.95 ± 0.22ab |
| 360 | 41.74 ± 1.42bc | 90.60 ± 5.26abc | 24.36 ± 1.02b | 44.97 ± 2.18bcd | 67.99 ± 1.89cd | 8.42 ± 0.29a | |
| 120 | 38.88 ± 1.19cd | 101.70 ± 3.05a | 14.29 ± 0.49e | 46.06 ± 2.11abc | 72.76 ± 3.00bc | 7.18 ± 0.16bc | |
| 100% | 240 | 44.50 ± 1.43ab | 95.50 ± 5.08abc | 21.04 ± 1.03e | 49.34 ± 2.47ab | 75.39 ± 4.04b | 8.42 ± 0.41a |
| 360 | 47.28 ± 2.28a | 88.33 ± 3.04bc | 22.15 ± 0.82e | 50.55 ± 1.02a | 84.44 ± 5.76a | 8.65 ± 0.30a | |
Unequal letters within a column indicate a significant difference between the samples with a significance level of p < 0.05, n=3. EY, extraction yield; TPC, total phenolic content; TAC, total monomeric anthocyanin content; UAE, ultrasound-assisted extraction ACV, apple cider vinegar.
3.3. Characterization of extracts obtained by UAE
The UAE is well established in the chemical and food industry due to its remarkable impact on the acceleration of various processes. It is based on the principle of acoustic cavitation, a phenomenon that breaks down the cell walls within the plant matrix, facilitating the extraction of bioactive compounds (Kumar et al., 2021; Razola-Díaz et al., 2022).
The EY values for black elderberry fruit extracts obtained with UAE using 30% ethanol as extraction solvent ranged from 21.64% to 47.28%. The EY values increased continuously with higher sonication amplitude and longer extraction time, as shown in Table 3. Considering the TPC, there was no clear pattern showing the impact of prolonged time or increased amplitude on the TPC. The highest TPC was obtained in the ethanol extract sonicated for 120 s at an amplitude of 100%, with 101.70 mg GAE/g DE, while the ethanol extract with the lowest TPC was obtained in sonication for 120 s at an amplitude of 60%, resulting in a TPC of 85.49 mg GAE/g DE (Table 3). It is noteworthy that the elderberry ethanolic extract obtained by the UAE showed a similar or some higher TPC value to that of the SLE. To assess the superiority of one technique over the other, the extraction times must be compared. It is obvious that UAE, which required less than 360 s, was a much more efficient method compared to SLE, which required a significantly longer extraction (24 h), in the process where the same raw material and same extraction solvent were used. This underlines the considerable timesaving advantages that the UAE brings.
The present study also included the evaluation of the role of 30% ethanol in the isolation of TAC from elderberry fruit in relation to UAE. Table summarizes the effects of different sonication amplitudes and extraction durations on TAC extraction. The optimum TAC from elderberry fruits was achieved with an extraction duration of 120 s and a sonication amplitude of 60%, resulting in a considerable amount of 30.36 mg C3G per liter (L). Same as in the case of TPC no clear pattern showing the impact of prolonged extraction time or increased amplitude on the TAC was not noticed.
In the case of UAE where ACV 4 was applied as extraction solvent, the EY values ranged from 39.55% to 50.55% and also showed an upward trend with increasing amplitude and time, except for the sample with 60% amplitude and 240 s, but with no significant difference.
Employing ACV as a solvent in the UAE process, variations in TPC were observed depending on the amplitude and duration of sonication. The most remarkable TPC was achieved under a sonication amplitude of 100% and an extraction duration of 360 s, resulting in a remarkable value of 84.52 mg GAE/g DE. The lowest TPC was measured in the ACV extract resulting from the sonication amplitude of 60% at an extraction time of 120 s (see Table 3).
Significantly, the use of ACV 4 as a solvent in the SLE resulted in a TPC of 61.42 mg GAE/g DE, but the use of UAE not only resulted in the extracts with higher TPC values but also did so in the significantly shorter time span of 360 s compared to the 24 h long SLE.
Extraction using ACV 4 as solvent yielded a peak TAC concentration of 8.65 mg C3G/L from elderberry fruit using UAE for 360 s at 100% amplitude. Here the TAC was impacted by the prolonged sonication time and by more intensive amplitude. Interestingly, for ethanolic extracts, this was not the case. However, the use of ultrasound with an amplitude of 60% showed a striking positive correlation between amplitude and TAC. Overall, ethanol can be considered a more efficient solvent for the extraction of anthocyanins from elderberry fruit, especially at a sonication amplitude of 100% and an extraction time of 360 s, which resulted in an approximately 2.5–fold higher TAC if ethanol is applied as extraction solvent.
3.4. Monitoring parameter changes during the UAE
The dynamics of temperature change over time is an important process parameter in the UAE. This interplay of temperature and time is of immense importance as it profoundly affects the extraction process. In certain cases, temperature can be a limiting factor in the extraction process, mainly due to the presence of heat-sensitive compounds and, accordingly, the boiling point of the solvent used. Oancea (2021) conducted an insightful study on the influence of temperature on the degradation of anthocyanins in elderberry fruit. The results of the study showed that an aqueous extract exposed to a temperature of 70 ◦C retained about 80% of its anthocyanin content during a heating period of 3 h. In view of these potential degradation processes and depending on the compounds to be isolated, strict temperature monitoring is essential throughout the extraction process.
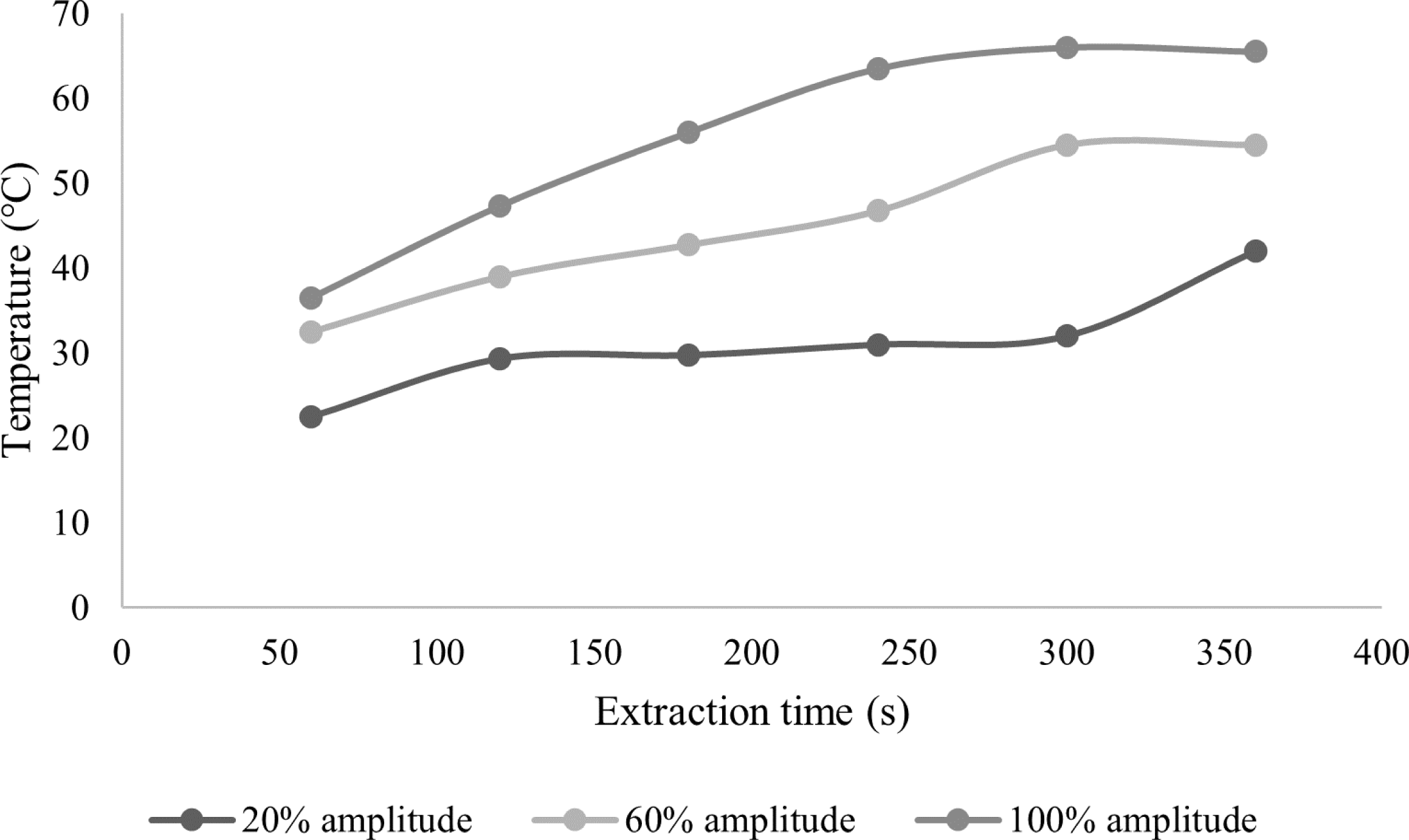
The relationship between extraction temperature and extraction time for elderberry extracts obtained with ethanol is shown in Figure 1, while Figure 2 shows the same correlation for elderberry extracts obtained with ACV. It is noteworthy that at a sonication amplitude of 20%, an initial temperature of 27 ◦C was measured, which increased slightly to 32.5 ◦C after 360 s of extraction. It is unlikely that this slight increase in temperature, especially in the lower temperature range, negatively affects the extracted polyphenolic compounds (Antony and Farid, 2022). Conversely, it could be assumed that this slight increase in temperature could even have a positive effect on the recovery of these compounds. At a sonication amplitude of 60%, a more pronounced increase in process temperature over time can be seen, and this increase is significant at an amplitude of 100%. Indeed, at this amplitude, the temperature starts at 30 ◦C and rises to 65.5 ◦C for 360 s. Obviously, in this scenario, the temperature variations during the extraction process are significant. This could either have a positive effect on the recovery of the compounds if the target compounds are thermostable, or a detrimental effect if they are susceptible to degradation at these high temperatures.
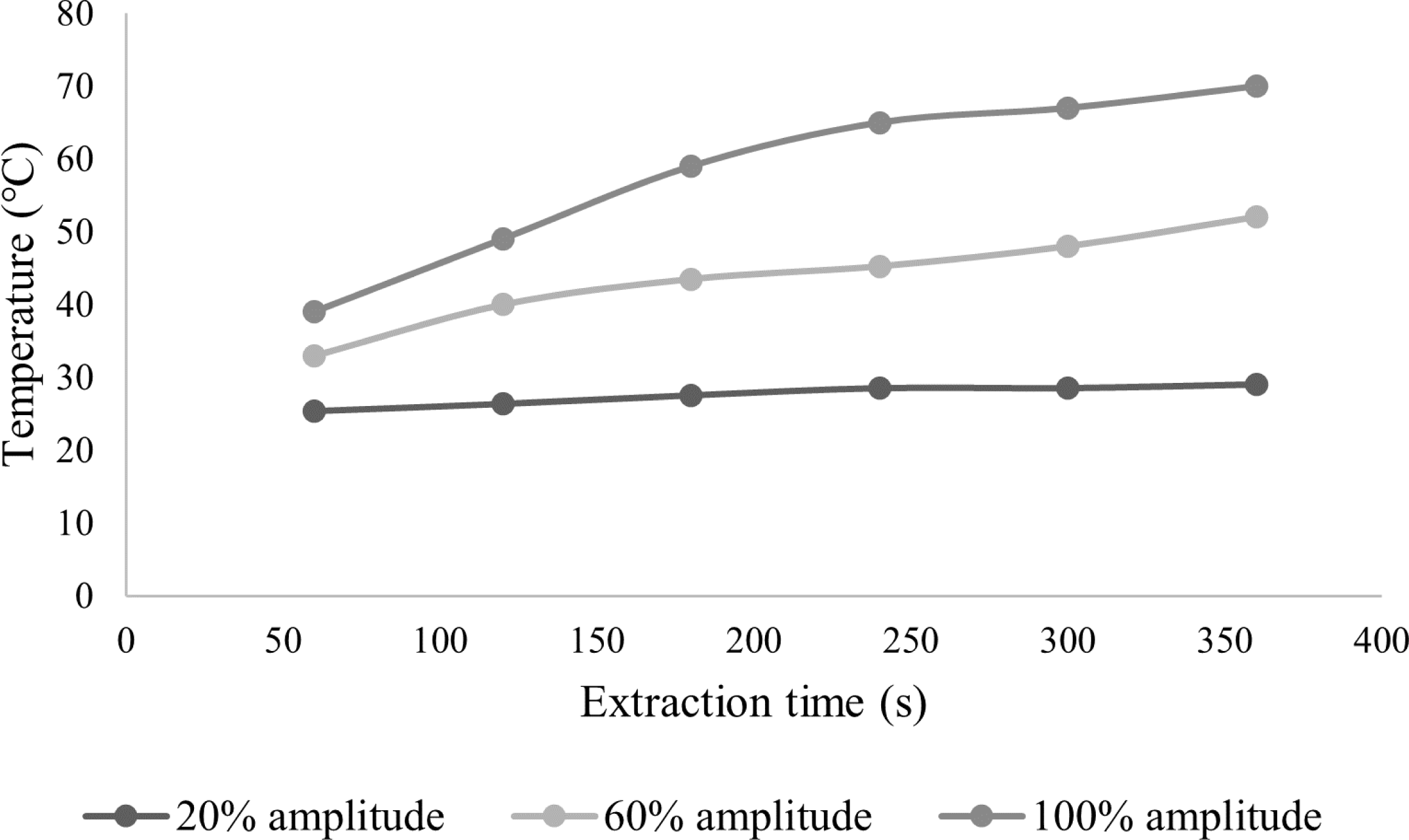
Figure 2 shows that the process temperature increases during the UAE with ACV as an extraction solvent. At a sonication amplitude of 20%, the process temperature was the lowest, 25.3 ◦C after 60 s and 29 ◦C at the end of the extraction process, after 360 s. It is important to note a gradual increase in temperature with time, but also with the amplitude of the extraction. Moreover, the temperature increases faster with time as the amplitude increases (Figure 2). At an amplitude of 100%, the temperature was 30.3 ◦C at the beginning of the extraction and 70 ◦C after 360 s.
Oancea et al. (2018) investigated the kinetics of anthocyanin degradation in elderberry fruit extracts. They concluded that conversions of anthocyanin compounds occur at temperatures above 100 ◦C. This could indicate that the highest temperature reached in this process had no negative effects on elderberry anthocyanins.
Energy consumption is an important process parameter in any production process and has a significant impact on the profitability and cost efficiency of the extraction process, especially when compared to alternative methods. Therefore, the evaluation of energy consumption is a critical dimension in the context of extraction processes. The results of the study showed a remarkable correlation between energy utilization and certain process variables. The lowest energy consumption of 0.50 W/h was observed at an extraction process amplitude of 20% and an extraction time of 60 s. In contrast, the highest energy consumption of 11.31 W/h was observed at an amplitude of 100% and an extraction time of 360 s. These results corresponded to the expected trends and confirmed the fundamental interplay between sonication parameters and energy consumption in UAE.
The study highlights an important result: increasing the sonication amplitude to 100% does not increase the efficiency of extraction, but leads to a significant increase in energy consumption of 33.4%. This has remarkable implications, especially for future extraction processes on an industrial scale. In addition to monitoring extract quality, it is crucial to evaluate the economic aspects, aiming for a double objective: optimizing extraction efficiency for resource utilization and minimizing energy consumption. This approach is crucial for cost efficiency and sustainable practices in large-scale production. In industrial production, every unit of energy consumed has a measurable economic impact, so examining the results of energy consumption is essential.
In the case of the ACV application, the results showed an increase in energy consumption over time that is in line with the trend observed in ethanol production. The lowest energy consumption was found to be 0.50 W/h at sonication amplitude of 20% for 60 s, while the highest energy consumption was measured at 12.30 W/h at a sonication amplitude of 100% for 360 s. This observation is in line with the results of Krivošija et al. (2023), who conducted a study on UAE using orange peel dust.
Therefore, it is advisable to opt for a lower sonication amplitude corresponding to the reduced output power to mitigate the significant 37% increase in energy consumption. This measure is in line with sustainable practices and responsible use of resources.
4. CONCLUSION
The results of this study provide convincing evidence for the effectiveness of ACV as an extraction solvent, particularly in facilitating the extraction of polyphenolic compounds, but this efficiency depends on the inherent properties of the ACV used. The highest TPC of 101.70 mg GAE/g dry extract (DE) was achieved by using 30% ethanol with a sonication amplitude of 100% and an extraction time of 120 s. Conversely, the use of ACV 4 resulted in the highest TPC, which reached 84.44 mg GAE/g DE with a sonication amplitude of 100% and an extraction time of 120 s. Indeed, ethanol proves to be a more effective solvent than ACV in every aspect. However, this does not diminish the significance of ACV application. The results emphasize the versatile utility of ACV as a solvent for the extraction of bioactive compounds and represent an important extension in the field of environmentally friendly extraction methods. The use of ACV as a solvent not only paves the way for novel, sustainable extraction methods, but also responds to today’s imperative to improve the environmental sustainability and efficiency of extraction processes in various scientific and industrial fields. Furthermore, the use of ACV (which in itself is a health-promoting, commercially available product) for the isolation of elderberry phenols enables the production of an elderberry product with strong quality characteristics that combine the health benefits of ACV and elderberry.