1. INTRODUCTION
Steppe peony (Paeonia tenuifolia L.), also known as the fern leaf peony, is a perennial, herbaceous plant species belonging to the Paeonia genus of the Paeoniaceae family (Li et al., 2021). On the basis of the phytochemical analyses of the petals of P. tenuifolia, a variety of biologically active compounds was identified, such as flavonoids, including anthocyanins and anthocyanidins, phenolic acids and terpenoids (Čutović, Batinić, Marković, Radanović, Marinković, Bugarski and Jovanović, 2022). Due to the presence of the mentioned components, steppe peony possesses a number of beneficial activities, such as antimicrobial, antibiofilm, and antioxidant properties, as well as the potential to enhance wound healing and inhibit the adhesion and invasion of the bacteria Staphylococcus lugdunensis, which is recognized as a skin pathogen (Čutović, Marković, Kostić, Gašić, Prijić, Ren, Lukić and Bugarski, 2022). On account of the fact that there is a limited bioavailability and instability of flavonoids, phenolic acids, and anthocyanins detected in the P. tenuifolia petal extracts (Fang and Bhandari, 2010), carriers for the extract should be prepared in order to protect the bioactive compounds and enable a more comfortable dermal application (de Vos et al., 2010). Plant extracts can be encapsulated into a matrix or membrane in the particulate form to achieve one or more of the previously listed desired effects (Armendáriz-Barragán et al., 2016). Encapsulation is intended to improve the stability of extracted compounds during processing, storage, or transportation. Its primary goal is to convert liquid active chemicals into solid forms in order to improve active compound management. When direct intake of an active substance affects human health, the use of pharmaceutical formulations containing them in an encapsulated form can be very advantageous. In addition, the encapsulation can also be used to improve the quality of the final product, isolate incompatible compounds, and provide the release of bioactives in a controlled way (El-Desoky et al., 2022).
Liposomes have been used as carriers for the delivery of various compounds, such as enzymes, polyphenols, drugs, proteins, vitamins, and antioxidants (Isailović et al., 2013; Jovanović et al., 2022). Liposomal preparation has several advantages over other kinds of encapsulation techniques, including liposome stability in products with a high water content and their capacity to encapsulate hydrophilic, amphiphilic, as well as lipophilic compounds (Jovanović et al., 2018). Additionally, after encapsulation, a controlled or sustained release profile can be achieved. Liposomes are also biodegradable and have a high affinity for cells (Lee et al., 1992). Further, the target impact of liposomal preparations has the power to modify the in vivo distribution of the loaded substance, therefore improving the biological effects of some treatments (Mohammed et al., 2004). Also, liposomes are non-toxic and biodegradable delivery carriers that are often made from naturally occurring substances, thus novel formulations might be easily deployed (Isailović et al., 2013).
The proliposome method provides a greater agitation energy input, resulting in smaller and more homogenous liposomes (Isailović et al., 2013). This procedure may be the simplest way to produce liposomes (Šturm and Poklar Ulrih, 2021). The key disadvantage is that, while it generates far greater encapsulation efficacy, it is not as repeatable when producing lower quantities of liposomes. During the production process of proliposome method, the compounds can be mixed with ethanol (in the case of lipophilic substances) or in an aqueous solution (in the case of hydrophilic substances). The utilization of proliposome technology opens up the possibility of large-scale liposome production (Elhissi et al., 2006).
No previous research was focused on the encapsulation of P. tenuifolia petal extracts into liposomal particles. Therefore, the goal of this study was to create P. tenuifolia petal extract-loaded liposomes and evaluate their antioxidant capacity, in order to potentially protect the sensitive biologically active components, preferably making them suitable for use in a variety of pharmaceutical and cosmetic formulations.
2. MATERIALS AND METHODS
2.1. Plant material and reagents
Steppe peony (P. tenuifolia) fresh petals were collected on a warm, sunny day in May of 2023 from spontaneously growing plants in their natural habitat in Gulenovci (840 m a.s.l.), Serbia. The Ministry of Environmental Protection of the Republic of Serbia has granted the license for their wild collecting (No. 353-01-121/2023-04, issued on March 3, 2022). The petals were hand-picked from full-blooming flowers at random. Less than 10% of the blooming plants discovered in the region were chosen to collect one-third of the petals per flower. The gathered petals were shade-dried at room temperature before being extracted.
Distilled water was purified through a Simplicity UV® water purification system (Merck Millipore, Merck KGaA, Germany). 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), methanol, potassium-persulfate, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were bought from Sigma-Aldrich (Taufkirchen, Germany), Phospholipon 90 G (a commercial lipid mixture, sunflower phosphatidylcholine from non-genetically modified plants, ≥ 90%) from Lipoid GmbH (Germany), liquid phospholipid mixtures Phosal 75 SA (containing phosphatidylcholine in ethanol and safflower oil, content ≥ 72.0%) and Phosal 53 MCT (phosphatidylcholine in medium-chain triglyceride, content ≥ 53.0%) were from Lipoid Skopje (Macedonia), ethanol (Fisher Science, UK), and potassium persulfate (Centrohem, Serbia).
2.2. Extraction of Plant Material
The biologically active components were extracted from the petals using the maceration method, by employing the linear mechanical homogenizer (Roller mixer SRT6, Potsdam, Germany) at room temperature (25 ± 5 ◦C) for 24 h using analytical grade methanol as the extraction solvent, with a solid-to-liquid ratio of 1:20. The extracts were filtered using a qualitative laboratory filter paper. Before further analysis, the obtained extract was evaporated to a dry mass at 30 ◦C in a drying oven (Sanyo drying oven MOV-212, Eschborn, Germany), after which they were kept in the dark at 4 ◦C.
2.3. Preparation of liposomes
The liposomes containing P. tenuifolia petal extract were prepared by employing the proliposome technique (Jovanović et al., 2022). In short, the liposomes were prepared using three phospholipids Phosal SA 75, Phosal MCT 53, as well as Phospholipon. At 50 ◦C, the phospholipids (4 g), analytical grade ethanol (15 mL), deionized water (3 mL), and dried petal extract (0.4 g) were mixed together. After the mixture had cooled to room temperature, deionized water (20 mL) was added in small amounts and the mixture was stirred at 800 rpm for the duration of 1 h.
2.4. Antioxidant potential of Paeonia tenuifolia petal extract-loaded liposomes
2.4.1. ABTS method
The ABTS assay was performed employing a slightly modified procedure that was previously described by Čutović, Batinić, Marković, Radanović, Marinković, Bugarski and Jovanović (2022). The stock solution of the ABTS•+ radical (7.8 mmol/L) was prepared by dissolving 20 mg of ABTS(s) in 5 mL of deionized water, followed by the addition of 88 μL of K2S2O8 solution with a concentration of 2.45 mmol/L (380 mg of potassium-persulfate(s) was dissolved in 10 mL of deionized water). The ABTS stock solution mixture was incubated for 16 to 20 h at 4 ◦C in the dark before use in order to activate the radical cation solution (ABTS•+). The obtained mixture was diluted with analytical grade ethanol to an absorbance value of approximately 0.700 at a wavelength of 734 nm. The precipitate obtained by the centrifugation of liposomes at 17500 rmp at 4 ◦C for 45 min was mixed with 200 μL of deionized water, in order to neutralize the amount of the supernatant drawn from the mixture. A sample (200 μL) was mixed with 2.8 mL of ABTS•+ solution and incubated for 30 min in the dark at room temperature. The measurements were performed in three parallel tries. The scavenging capacity was calculated as per Equation 1: (1) where Asample was the absorbance of ABTS•+ solution and the sample (the liposomes with P. tenuifolia petal extract), while Acont was the absorbance of the blank solution.
2.4.2. DPPH method
The DPPH radical scavenging activity was assessed using method previously described by Blois (1958). The DPPH solution was prepared by combining 9 mL of ethanol with 0.252 mg of DPPH. The liposomal suspension (200 μL) was combined with 2.8 mL of this solution, and the mixture was then left to incubate in the dark, at room temperature for 30 min. The scavenging activity (SCDPPH) was calculated using the following Equation (2) after the absorbance was measured at 517 nm: (2) where Acont represents the absorbance value of the blank solution, while Asample is the absorbance of the liposomal suspension sample treated with the DPPH ion solution.
2.5. Statistical analysis
The statistical analysis was performed using the analysis of variance (one-way ANOVA) followed by Duncan’s post hoc test, within the statistical software STATISTICA 7.0. The differences were considered statistically significant at p < 0.05, n=3.
3. RESULTS AND DISCUSSION
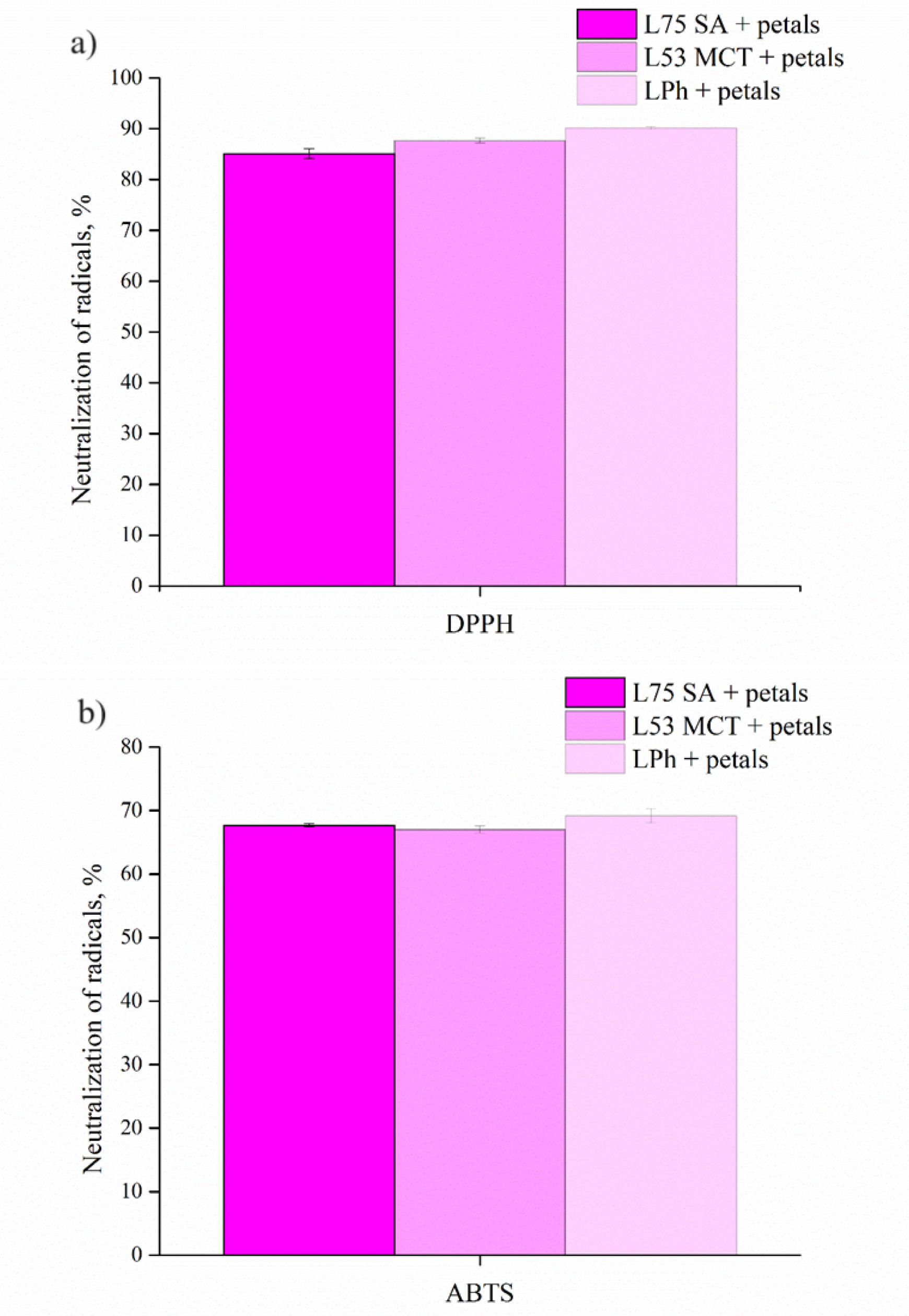
In this study, the antioxidant potential of the P. tenuifolia petal dry methanolic extract-loaded liposomes, prepared using the proliposome method, was measured using two radical scavenging assays, ABTS and DPPH. The results of the antioxidant activity of the obtained liposomes are presented in Figure 1. L75 SA + petals – liposomes composed of Phosal 75 SA phospholipid mixture, and the petal extract, the L53 MCT + petals liposome contains Phosal 53 MCT and the petal extract, while LPh + petals, consists of Phospholipon and the petal extract. As can be seen from Figure 1, the ABTS radical neutralization capacity of the LPh + petals liposome was the highest, with 69.15 ± 1.14% of radical neutralization, followed by L75 SA + petals liposome at 67.68 ± 0.24%, which were similar to the L53 MCT + petals liposomes (66.99 ± 0.54%). The DPPH antioxidant potential of the liposomes followed a similar trend, with the highest level of DPPH ion neutralization achieved by LPh + petals liposomes (90.11 ± 0.3%), and L75 SA + petals being the least effective (85.12 ± 0.94%). The reason why LPh + petal liposomes showed the best ability to neutralize both ABTS+ and DPPH ions could be attributed to difference in the composition of the lipid mixtures, as Phospholipon is a solid lipid mixture of sunflower phosphatidylcholine, whereas Phosal 75 SA and Phosal 53 MCT are liquid, and contain phosphatidylcholine in ethanol and safflower oil/or medium-chain triglyceride, thus making the bilayer of LPh + petals liposomes more permeable.
The antioxidant potential of pure methanolic P. tenuifolia petal extract has been a subject of our previous work, and it has been shown that the IC50 value for the neutralization of DPPH ions was 0.123 ± 0.001 mg/mL, whereas for the ABTS+ ions was 0.099 ± 0.000 mg/mL, thus, when incorporated into liposomes, their antioxidant ability was changed, which may be due to the interactions between the lipids and the extract (Čutović et al., 2023) during the preparation of liposomes, as the bioactive compounds responsible for the neutralization of the ABTS+ ions could possibly be encapsulated more efficiently, than the compounds that have the ability to neutralize DPPH ions in the in vitro assay (Nikolic et al., 2018). The chemical composition of the methanolic P. tenuifolia petal extract has been previously assessed (Čutović, Batinić, Marković, Radanović, Marinković, Bugarski and Jovanović, 2022), and it has been shown that the extract is rich in phenolic acids and flavonoids, which are compounds widely know for their antioxidant potential (Kim et al., 2012).
The comparison of results in this study to those from the literature is not always possible, due to the difference in their expression (%, mg of antioxidant compound equivalent/ mg or mL of extract, etc.), and methodology.
The obtained results for the ABTS radical scavenging activity for all three extract-loaded liposome types were higher than in the case of rosehip extract-loaded liposomes, where the neutralization of ABTS+ radicals was 45.4 ± 1.8% (Jovanović et al., 2023), which is more than 20% lower than in the case of the least efficient P. tenuifolia petal extract-loaded liposome, probably due to differences in composition of the extracts, the content of compounds with antioxidant potential, and used phospholipid mixtures. In other study (Alemán et al., 2019) the ABTS assay was performed on the freeze-dried liposomes containing Crithmum maritimum leaves and stems aqueous and ethanolic extracts, and the results were 556.8 ± 4.26 mg vit C eq./g, and 805.1 ± 7.37 mg vit C eq./g, respectively. These results are not comparable to the ones presented in this study, as in this study liposomes were tested right after the preparation whereas theirs were lyophilized, and the expression units also differ. In case of DPPH radical scavenging, the results from this study were higher than in the research with rosehip extract-loaded liposomes (Jovanović et al., 2023), where the neutralization was 57.7 ± 1.7%, probably due to the difference in the chemical composition of the extracts, as well as the encapsulation efficiency of extracts into liposomes. On the other hand, in the work of Dag and Oztop (2017) the antioxidant activity of green tea extract-loaded liposomes determined by the DPPH assay was in the range from 15.051 to 20.451 mg DPPH/L sample, but the results are again not comparable due to differences in expression units.
4. CONCLUSION
In the present research, liposomes containing the dry methanolic extract of P. tenuifolia petals, endangered wild growing plants from Serbia, have been developed and characterized from the standpoint of their antioxidant activity, by the radical scavenging methods. The liposomal particles showed a very high level of both the ABTS and DPPH radical scavenging. However, the extract-loaded liposomes have shown to be more efficient in scavenging DPPH ions, due to the presence of antioxidants in the petal extract, more capable of interacting with them, than with ABTS+. The lipid mixture that was used for the preparation of the liposomes with the highest antioxidant capacity is Phospholipon, due to the difference in its chemical composition, in comparison to the other two phospholipid mixtures. This was the first step in assessing the biological activities of P. tenuifolia petal extract-loaded liposomes, so further study should be focused on other biological activities for their potential industrial application.