1. INTRODUCTION
Today, the cosmetics industry represents a growing part of the economy, with a focus on cosmetic products based on environmentally-friendly natural ingredients. This trend is driven by the global transition to a “greener” lifestyle, and so the demand for natural ingredients is increasing. Polyphenolic compounds, essential oils, and plant extracts are natural ingredients that are broadly used in the cosmetics industry (Piccolella et al., 2019). These compounds with proven antioxidant, anti-inflammatory, and soothing properties, could increase the value of cosmetic products, especially skin-care products. Although natural-based cosmetics contain a large amount of bioactive nutrients, they could also disrupt product stability (Rybczyńska-Tkaczyk et al., 2023). Moreover, high water content in cosmetic products, such as creams, favors the development of pathogenic microorganisms, that could affect the quality of products, as well as human health (Barthe et al., 2021). Substances with antimicrobial properties, used as preservatives in cosmetics, could ensure their shelf life and safety (Campana et al., 2006). Despite their function, the addition of preservatives can be considered as one of the most controversial issues in the cosmetics industry. Recently, there has been a growing interest in replacing synthetic chemical preservatives with natural ones, as the increasing use of natural compounds in cosmetic products has raised customer awareness of the negative effects of synthetic preservatives (Rybczyńska-Tkaczyk et al., 2023). Therefore, effective and low-toxicity preservative compounds without side effects should be imperative in modern cosmetology (Rybczyńska-Tkaczyk et al., 2023).
Parabens are synthetic preservatives used in various industries since 1920. They are derived from synthetic esters of p-hydroxybenzoic acid (methylparaben, ethylparaben, n-propylparaben, benzylparaben, isobutylparaben, isopropylparaben, n-butylparaben and heptylparaben and their respective sodium salts) (Nowak et al., 2021). Unfortunately, when excessive quantities of cosmetic preparations containing these compounds were used, adverse health outcomes of parabens have been reported and some studies speculated that parabens demonstrated side effects on the endocrine system and even diseases such as cancer, thyroid disorders, skin allergies, or even reproduction and neurological problems (Barthe et al., 2021; Matwiejczuk et al., 2020; Mitra et al., 2021). Despite reports found in literature causing bad marketing reputation of parabens, according to the valid regulatory directives concerning cosmetic (European Commission, n.d.) usage of some parabens such as methylparaben, propylparaben, butylparaben, and ethylparaben is permitted, while some parabens are banned for use in cosmetic products.
Day and Night creams (Neven dnevni krem® and Neven noćni krem®) with marigold flowers (Calendula officinalis L., Asteraceae) extract have been produced by the Institute for Medicinal Plants Research “Dr. Josif Pančić” (Belgrade, Serbia), for more than 10 years. Marigold is an annual herbaceous plant abundantly distributed in Central Europe, Middle Eastern countries and the Mediterranean regions, with a long history of use in traditional medicine (Shahane et al., 2023). The 10th European Pharmacopoeia states that ligulate flowers (Calendulae flos), which have been detached from the receptacle, represent drug intended for pharmaceutical use. Phytochemical studies of marigold flowers have found that the most important constituents are triterpene pentacyclic alcohols (dominantly faradiol derivatives) and triterpene saponins (calendasaponins A, B, C and D), along with polysaccharides, carotenoids (lutein and zeaxanthine), flavonoids (hyperoside and rutin), coumarins and essential oil (Barnes et al., 2007; Heinrich et al., 2017; Shahane et al., 2023). These pharmacologically active principles could be connected with antioxidant, anti-inflammatory, antimicrobial and wound-healing activities of marigold, which have been demonstrated in numerous in vitro and in vivo experiments (Barnes et al., 2007; Shahane et al., 2023). On the basis of these attributes, the Committee on Herbal Medicinal Products (HMPC) of the European Medicines Agency (EMA) approved marigold flowers to be used in different forms of traditional herbal medicinal products for the symptomatic treatment of minor inflammations of the skin, as well as aid in the healing of minor wounds.
However, previously mentioned Day and Night skin care formulations were manufactured and preserved using the mixture under the trade name Dekaben C® (INCI Name: Phenoxyethanol, Methylparaben, Ethylparaben, Butylparaben, Isobutyl paraben, Propylparaben). Prohibition of isobutylparaben usage, one of the ingredients of the used preservative mixture, according to the Cosmetic Directive by European Commission, from the regulatory safety aspect, imposed reformulation of stated products, in terms of preservative replacement.
For this purpose, Day and Night creams were preserved with Gujsol-1® (INCI name: Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben, Butylparaben) or potassium sorbate instead of preservative Dekaben C®. Gujsol-1® was chosen due to the similarity to the previously used Dekaben C®, but without the presence of the unacceptable isobutylparaben. Potassium sorbate is a potassium salt of sorbic acid, widely used as a preservative in cosmetic products. This cosmetic ingredient provides prolonged shelf life of the product by inhibition of the growth of mold, yeast, and bacteria, and thus prevents spoilage and ensures consumer safety. Considering that it represents salt of unsaturated fatty acid that participate in the normal human fat metabolism. Therefore, it is oxidized into carbon dioxide and water, without accumulation in the human body (Jorge, 2003).
Bearing in mind that preservatives have a crucial influence on the microbiological stability of cosmetic products, comprehensive stability study was carried out in order to evaluate influence of different preservatives (Gujsol-1® and potassium sorbate) on Day and Night skin care formulations with marigold extract.
2. MATERIALS AND METHODS
2.1. Creams preparations
Two types of formulations were prepared, Day Cream with marigold extract-DC and Night Cream with marigold extract- NC, both prepared with two different preservatives, Gujsol-1® (DC-1 and NC-1, respectively) and potassium sorbate (DC-2 and NC-2, respectively).
Marigold propylene glycol extract (0.5 %, w/v) (1:4, solid: solvent ratio) and rose hip propylene glycol extract (0.3 %, w/v) (1:4, solid: solvent ratio), obtained from the production sector of the Institute for Medicinal Plant Research “Dr. Josif Pančić” (Belgrade, Serbia), were used for creams preparations. Extracts were prepared by percolation method with propylene glycol (45%) as a solvent instead of alcohol, as propylene glycol has a beneficial effect on the skin and it is well tolerated in the cream formulations. In addition to both formulation ’s composition the following active components were incorporated: avocado oil, shea butter, elastin protein, vitamin E, natural wetting factors. Night cream additionally contained glycine soja (soybean) seed extract. The formulation was made without alcohol addition, paraffin and synthetic dyes in order to grease the skin and impair the natural skin barrier. Day and Night skin-care creams were prepared by continuous mixing of the oil and aqueous phases. Active compounds were added at a temperature below 45 ◦C in order to preserve the stability of the sensitive active compounds, such as avocado oil, a mixture of extracts, vitamin E, natural moisturizing factor, and glycine soja (soybean) seed extract. Day and Night formulations with marigold extract, assigned as DC-1, NC-1, were prepared with Gujsol-1® (1%) as preservative, while formulations DC-2 and NC-2 were preserved with and potassium-sorbate (0.6%). Formulations DC-1 and DC-2, as well as NC-1 and NC-2 were prepared in laboratory conditions, while control samples (DC-control and NC-control) were prepared by the standard procedure in the production sector of the Institute for Medicinal Plants Research “Dr. Josif Pančić” (Belgrade, Serbia) using Dekaben C® as preservative.
2.2. Stability tests
Stability tests of prepared formulations were performed on both series of reformulated skin care creams with Gujsol-1® (series DC-1 and NC-1) and potassium sorbate (series DC-2 and NC-2), as well as samples with the Dekaben C® (DC-control and NC-control). Samples were filled in the original packaging and divided into three groups, for the purpose of stability tests according to the following protocol:
2.2.1. 1) Storage at room temperature (during the expected shelf life)
The stability of the samples was evaluated after 24 hours after preparation and then after 7, 19, 90, 180, 270 days, 12, 18 and 24 months of storage of the samples at 25 ± 2 ◦C.
2.2.2. 2) Accelerated storage with increased temperature
The stability of the samples was monitored after the sample’s storage at increased temperature conditions (40 ± 2 ◦C), without light in the thermostat chamber. The samples were analyzed after 90 and 180 days.
2.2.3. 3) Cyclic stress test
Samples were exposed to a cyclic stress test, where formulations were evaluated initially after preparation and after 18 days. This test was performed 24 hours after samples preparation, and samples were exposed to three different temperature conditions, i.e., at reduced temperature (4 ± 2 ◦C), room temperature (25 ± 2 ◦C) and increased temperature (40 ± 2 ◦C), following six cycles in total.
2.3. Formulation analysis
Organoleptic properties, pH, and physical stability were monitored at defined time points during the stability tests.
2.3.1. Organoleptic characteristics
The color, consistency and homogeneity of the samples were monitored by visual evaluation in the package, by application and smearing on a glass plate. Application characteristics (lubricity, appearance, shine of the smear on the skin, penetration (absorption) of the cream into the skin, subjective feeling of hydration, dryness/oiliness and occlusion) were monitored after applying the product to the skin.
2.3.2. Measurement of pH value
This analysis was performed by the potentiometric method by pH meter (Hanna instruments HI 99161, Portugal), directly in the prepared samples, by immersing the electrode of the pH meter in the tested samples. Before measurements, the apparatus was calibrated with standard buffers pH 7.0 and pH 4.0.
2.3.3. Centrifugal tests
An adequate mass of formulations was put into plastic cuvettes, and then subjected to centrifugation (3000 rpm, for 30 min), in order to examine potential separation of the water and oil phases (Centrifuge, Hermle Z206 A).
2.4. Microbiological stability of skin-care formulations
Prepared creams should be applied on face skin and thus should meet certain requirements regarding microbiological integrity (purity), according to the Regulation on Cosmetic Products ( Official Gazette of RS, 60/2019, 47/2022, 21/2023, 2023). According to this Regulative, microbiological quality acceptance criteria required in cosmetic products for adults should be:
- the total number of aerobic microorganisms (bacteria plus yeast and molds) should not be greater than 103 CFU/g or CFU/mL
Candida albicans Absence in 1 g or 1 mL
Staphylococcus aureus Absence in 1 g or 1 mL
Pseudomonas aeruginosa Absence in 1 g or 1 mL
Escherichia coli Absence in 1 g or 1 mL
2.5. Challenge tests
A test of the preservative’s effectiveness (challenge tests) of the tested formulations was also performed. The antimicrobial properties of the preservative are considered adequate if under the test conditions, there is a significant decrease or no increase in the number of microorganisms in the inoculated preparation after the defined period.
The test microorganisms for analysis were selected according to the recommendation of the ISO 11930 standard:
Bacteria:
Escherichia coli ATCC 8739
Pseudomonas aeruginosa ATCC 27853
Staphylococcus aureus ATCC 25923
Yeast:
Candida albicans ATCC 10231
Mold:
Aspergillus brasiliensis (A. niger) ATCC 16404
Creams inoculated with calibrated inoculum, which was prepared from relevant ATCC strains were evaluated. Suspensions of selected microorganisms in the inoculum for contamination were made in adequate liquid media (Mueller Hilton Broth and Tryptone Soya Broth). After overnight incubation, the number of cells in the inoculum was measured by spectrophotometric method via optical density (o.d.). The number of cells in the suspension was adjusted for both bacteria (107 CFU/mL), yeast and mold (from 104 to 106 CFU/mL). Successive decimal dilutions of the calibrated suspension were made in the diluent. The number of test microorganisms in the suspension was determined by seeding 1 mL of the appropriate decimal dilutions in duplicate on the appropriate substrates. The plates were incubated at 32 ± 2.5 ◦C for 48 h (bacteria and C. albicans) and at 25 ± 2.5 ◦C for 5 days for A. brasiliensis.
According to the ISO Standard 11930:2019 procedure, calibrated inoculum (0.2 mL) was added to cream formulations (20 g) to obtain a certain number of microorganisms in the final concentration. In addition, inoculation with mixed cultures of all test microorganisms was also performed, in duplicate. All creams were mixed after inoculation to ensure a homogeneous distribution of the inoculum. The inoculated creams were stored in the premises of the microbiological laboratory of the Institute for Medicinal Plants Research “Dr. Josif Pančić” (Belgrade, Serbia), where analyses were performed at each specific interval in accordance with the ISO Standard method. Samples were stored at room temperature (25 ◦C) which support the growth of microorganism and their possible reaction with preservative in the samples.
Before starting the test, it was necessary to check zero, i.e. initial determination of the microbiological purity (integrity) of the prepared samples.
The number of viable microorganism’s existent in creams was performed at specific intervals in accordance with each strain, 7, 14, 28 days after inoculation, by plate count method. The microbiological stability of the creams and the antimicrobial potential of the investigated preservatives were checked even after 50 days of storage. According to the regulations, microbiological stability of the creams and the antimicrobial potential of the investigated preservatives, Gujsol-1® and potassium sorbate should be considered appropriate if the abundance of all microorganisms decrease in all tested intervals.
3. RESULTS AND DISCUSSION
3.1. Organoleptic characteristics
According to the results of organoleptic analysis, all tested formulations were white to light orange homogeneous, glossy creams, with a pleasant, mild smell. Moreover, all creams were easily spread on the skin, leaving a gentle feel. It was evident that during real storage and cyclic temperature stress tests, there were no changes in the organoleptic characteristics of creams (Figures 1 and 2). On the other hand, during storage under increased temperature a slight change of creams color (i.e., slightly darker color) was observed. It is known that color changes could be caused by chemical reactions (oxidation, reduction and hydrolysis), as well as chemical interactions between formulation ingredients, which are particularly induced at high temperature conditions (Kamaruzaman and Yusop, 2021).
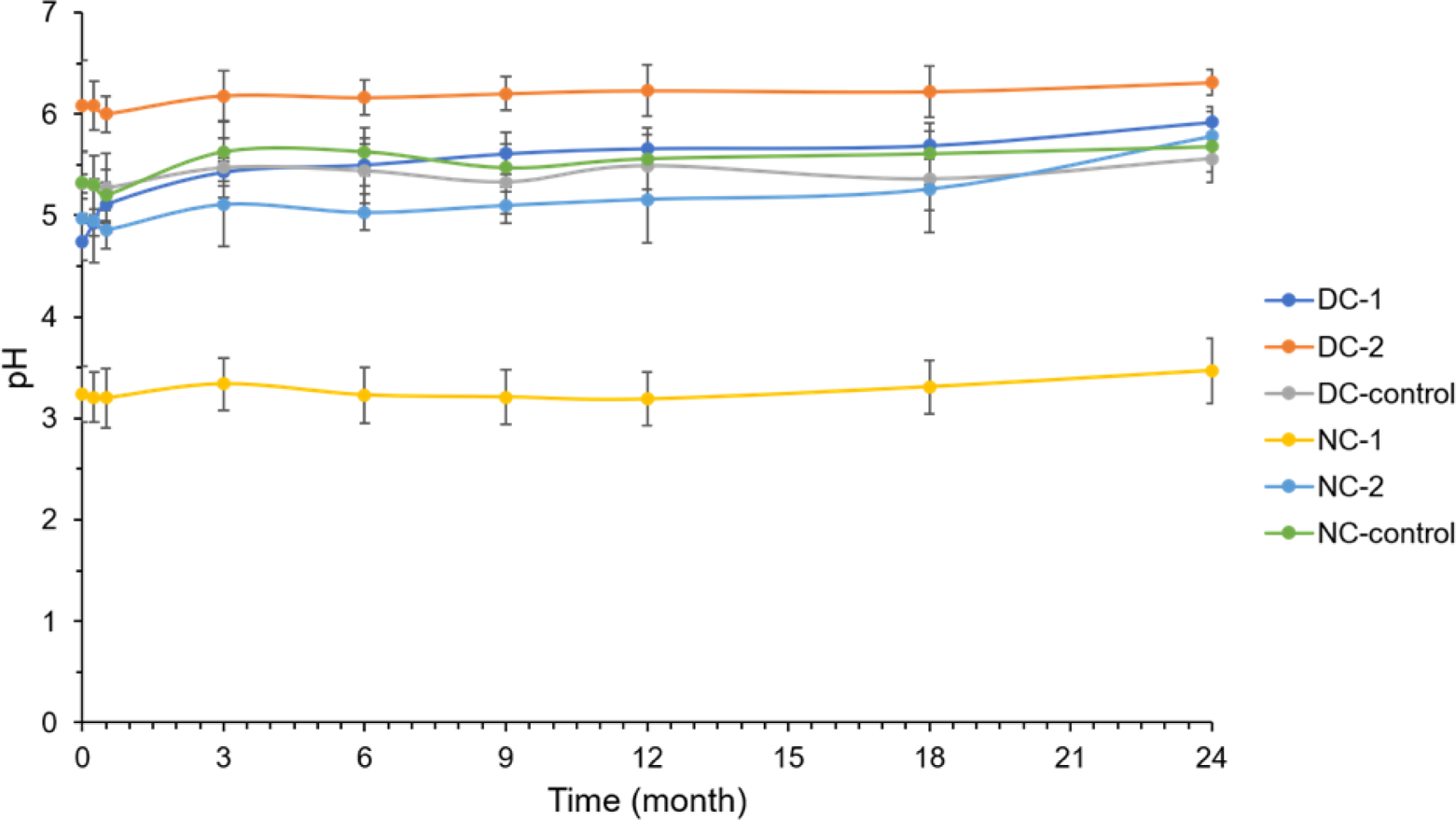
3.2. Evaluation of pH value
With regard to the effectiveness and stability of preparations intended for topical applications, the pH value of creams represents an important parameter that should be determined. In the current Book of cosmetic products ( Official Gazette of RS, 60/2019, 47/2022, 21/2023, 2023), the pH value of the cosmetic product should be in the range of 3.0 to 10.0. It has been established that the skin surface has a mild acidic pH, within the range of 4.0 to 6.0, which is associated with a number of physiological roles, such as defense mechanism against harmful microorganisms and activation of enzymes responsible for the skin barrier function (Buraczewska and Lodén, 2004). The results of the pH values of the examined creams are shown in Table 1 and Figure 3. Generally, the pH values of the creams differed in dependence on the formulation, while storage conditions had a low impact on this parameter. The lowest pH value was estimated for NC-1 (3.22) whereas the highest pH value was determined for DC-2 (6.08), which can be ascribed to intrinsic characteristics of preservatives. Namely, in case of Gujsol-1® contained creams, DC-1 and NC-1, the measured pH values were lower in comparison to creams with potassium sorbate, DC-2 and NC-2. During the storage of samples at room (25 ± 2 ◦C) and elevated (40 ± 2 ◦C) temperature, a mild increase of pH values was observed. The noticed changes in pH values may be due to the chemical reaction which has been occurred between the ingredients of creams, followed by formation of different classes of chemical compounds (aldehydes, acids, alcohols). Moreover, literature data indicate that fluctuations in the pH could be affected by nature of plant extract, temperature at the time of the measurement of pH and product contamination (Kamaruzaman and Yusop, 2021; Sathya et al., 2017). Also, after the cyclic temperature stress test, almost the same level of pH was measured in creams compared to the initial point.
| Cyclic stress test | Accelerated storage | ||||
|---|---|---|---|---|---|
| 0 days | 18 days | 0 days | 90 days | 180 days | |
| DC-1 | 4.74 ± 0.18 | 5.31 ± 0.15 | 4.74 ± 0.18 | 5.88 ± 0.21 | 6.01 ± 0.21 |
| DC-2 | 6.08 ± 0.21 | 5.99 ± 0.13 | 6.08 ± 0.21 | 6.17 ± 0.41 | 6.11 ± 0.28 |
| DC-control | 5.32 ± 0.13 | 5.41 ± 0.29 | 5.32 ± 0.13 | 5.87 ± 0.26 | 5.91 ± 0.16 |
| NC-1 | 3.22 ± 0.18 | 3.13 ± 0.11 | 3.24 ± 0.18 | 3.30 ± 0.17 | 3.48 ± 0.14 |
| NC-2 | 4.97 ± 0.29 | 5.72 ± 0.17 | 4.97 ± 0.29 | 5.71 ± 0.19 | 5.68 ± 0.31 |
| NC-control | 5.33 ± 0.12 | 5.41 ± 0.25 | 5.33 ± 0.12 | 5.69 ± 0.14 | 5.80 ± 0.08 |
3.3. Centrifuge assay
In order to evaluate the physical stability of creams, a centrifuge assay was performed. The centrifugation test is simulating the influence of gravity on the investigated samples and no signs of phase separation or other physical instabilities after centrifugation were found in any of the tested samples obtained during real storage, accelerated storage, and cyclic temperature stress testing. The results obtained in this assay indicate that creams have satisfactory physical stability during 24 months at room temperature, as well as during 6 months at elevated temperature.
3.4. Microbiological stability
A key aspect of cosmetic products’ safety is related to their microbiological quality and stability. Cosmetic products should not represent a potential risk to the customer’s health throughout their shelf time period. Although cosmetic products are not sterile, they should not be contaminated with pathogenic microorganisms, and the content of non-pathogenic (saprophytic) microorganisms must be at an appropriate (low) level (Kim et al., 2020).
The microbial contaminants of cosmetics can originate from a) contaminated raw materials, b) the specific production process of cosmetics, c) the sanitary conditions of the environment and equipment (i.e., improper air conditioning, reuse of utensils, etc.) as well as d) poor personal hygiene. Water and raw materials of animal, plant, and mineral origin per se are among the main sources of bacterial contamination. Moreover, consumers store most cosmetic products in the bathroom, where the temperature and humidity are elevated, which affects the development of microorganisms (Kim et al., 2020; Krajišnik and Ðekić, 2018; Neza and Centini, 2016).
Several studies have shown that the most frequently found microorganisms in cosmetics are P. aeruginosa, S. aureus, E. coli, and Bacillus species, as well as other bacteria, mold, and yeasts (Budecka and Kunicka-Styczyńska, 2014). Fungal multiplication in cosmetics is more rapid and evident than bacteria, and fungal infections can be caused by Candida, Aspergillus, and Penicillium species. Microorganisms such as P. aeruginosa, S. aureus, C. albicans and A. brasiliensis were restricted by EU Pharmacopoeia as most founded contaminants pretentiousness microbial spoilage in cosmetics and risk to the consumer health.
The consequence of the growth and reproduction of microorganisms in cosmetic products can cause degradation of ingredients and disruption of the organoleptic characteristics of the products that manifest as a visible presence of microorganisms and/or the appearance of an unpleasant odor, change in pH, texture, viscosity or color, discoloration, destruction of emulsion systems (Orus and Leranoz, 2005). Microorganisms can survive in harsh conditions and are resistant to various antimicrobial agents. Since microorganisms are ubiquitous in nature, they can be easily transported to the cosmetic environment through human hands.
The current European regulatory framework in the field of cosmetic products requires manufacturers to provide results of preservative efficacy testing (challenge test), as well as to ensure microbiological stability data that are documented in the safety report. It is also important to identify the microbiological stability and durability of cosmetics through the storage period and to determine the potential risk of contamination during consumer use.
In our study, creams with preservatives potassium sorbate and Gujsol-1® were experimentally evaluated in order to investigate the microbiological stability of the DC-1, DC-2, DC-control, NC-1, NC-2, NC-control during storage and application. Day and night skin-care formulations with both tested preservatives were microbiologically stable immediately after production as well as in each tested point after storage for a certain period at elevated temperature, and during cyclic stress test. At the zero point of the test, immediately after the formulation production, a certain but allowed total number of aerobic mesophilic microorganisms (bacteria plus yeasts and molds) was detected in DC-2 (Table 2). In the following testing points their number was completely reduced by the activity of preservatives. Furthermore, all investigated formulations stored at room temperature were microbiologically stable for two years.
| Type of testing | Reference values | DC-1 | DC-2 | NC-1 | NC-2 |
|---|---|---|---|---|---|
| Total aerobic mesophilic microorganisms (bacteria plus yeasts and molds) | <103 CFU/g ili mL | <102 | 2x102 | <102 | <102 |
| Candida albicans | absence in 1 g or 1 mL | not found | not found | not found | not found |
| Pseudomonas aeruginosa | absence in 1 g or 1 mL | not found | not found | not found | not found |
| Escherichia coli | absence in 1 g or 1 mL | not found | not found | not found | not found |
| Staphylococcus aureus | absence in 1 g or 1 mL | not found | not found | not found | not found |
This result has indicated that Day and Night skin-care formulations, with both preservatives, potassium sorbate and Guisol-1®, had good microbiological quality during the entire period of storage (2 years). Regardless of whether the formulations were exposed to room temperature, elevated temperature or cyclic stress test conditions, as previously explained, the applied preservatives provided them excellent microbiological quality throughout the test period.
3.5. Challenge tests
Given that EU Regulation 1223/2009 does not provide recommendations for a specific test that can be used to test the effectiveness of preserving cosmetic products (preserving microbiological purity), several tests are in use: Pharmacopoeia (Ph. Eur. 11.0), ISO 11930, Koko test, etc. Generally, ISO Standard 11930 (“Evaluation of the antimicrobial protection of a cosmetic product” or “Challenge test”) is an overall standard, which is used to evaluate the anti-microbial stabilization of a cosmetic product.
Before the Challenge test performance, the microbiological quality of the samples was checked according to the Regulation on cosmetic products ( Official Gazette of RS, 60/2019, 47/2022, 21/2023, 2023), which was zero point of initial examination. According to this Regulation, the number of certain microorganisms was determined before treatment (initial - zero test), presented in Table 2. The obtained results indicated that all creams were microbiologically approved. The conservation efficiency test is based on the “provoking” formulation in the final packaging by suitable microorganisms’ inoculum. Namely, after the storage of inoculated preparation at a prescribed temperature, samples were analyzed at certain time intervals by counting microorganisms detected in the samples. The antimicrobial properties of the preparation are adequate if, in the test conditions, there is a significant decrease or no increase in the number of microorganisms in the inoculated preparation after the examination period at the defined temperatures.
The results of the tests at seven-day intervals are shown in Table 3. After seven days of inoculation, the presence of all microorganisms in a certain number in both cream formulations with both preservatives was confirmed. After 14 days of storage, the complete absence of E. coli and P. aeruginosa was established in the Day and Night creams due to the action of both tested preservatives, Gujsol-1® and potassium sorbate.
| Test microorganisms | Initial inoculum | Obtained values | |||
|---|---|---|---|---|---|
| DC– 1 | DC – 2 | NC– 1 | NC – 2 | ||
| Escherichia coli ATCC 8739 | 107 | ||||
| After 7 days | 2x103 | 2x104 | 3x103 | 104 | |
| After 14 days | / | / | / | 2x102 | |
| After 28 days | / | / | / | / | |
| Pseudomonas aerugin ATCC 27853 | 2x107 | ||||
| After 7 days | 2x102 | 102 | 2x102 | 3x102 | |
| After 14 days | / | / | / | / | |
| After 28 days | / | / | / | / | |
| Staphylococcus aureu ATCC 25923 | 3x107 | ||||
| After 7 days | 3x104 | 5x105 | 2x104 | 105 | |
| After 14 days | 2x102 | 4x103 | 2x102 | 2x104 | |
| After 28 days | 20 | 20 | 30 | 5x103 | |
| After 50 days | / | / | / | / | |
| Candida albicans ATCC 10231 | 2x106 | ||||
| After 7 days | 3x103 | 2x103 | 5x103 | 2x104 | |
| After 14 days | 5x102 | 2x102 | 3x102 | 3x103 | |
| After 28 days | 102 | <102 | <102 | 102 | |
| After 50 days | / | / | / | / | |
| Aspergillus brasiliens ATCC 16404 | 104 | ||||
| After 7 days | 103 | 2x103 | 102 | 3x102 | |
| After 14 days | 5x102 | 4x102 | 2x102 | 102 | |
| After 28 days | 2x102 | 102 | 102 | <102 | |
| After 50 days | 10 | <102 | 10 | / | |
| Mixed inoculation | |||||
| After 7 days | 5x 103 S. aureus
102 P. aeruginosa 7x103 C. albicans 6x102 E. coli |
3x102 A. niger
5x103 E. coli 102 P. aeruginosa 7x103 C. albicans |
>104 S. aureus
2x102 A. niger 103 C. albicans 2x102 A. niger |
2x102 A. niger
2x103 E. coli 6x103 C. albicans 2x102 P. aeruginosa |
|
| After 14 days | 7x102 A. niger
5x 103 S. aureus 3x102 C. albicans 2x102 A. niger |
5x 103 S. aureus
5x102 S. aureus <102 A. niger |
<102 P. aeruginosa
4x103 S. aureus; 102 A. niger; |
3x104 S. aureus
<102 E. coli 104 S. aureus |
|
| After 25 days | 102 C. albicans
30 S. aureus |
20 S. aureus
102 A. niger |
103 S. aureus | 103 S. aureus | |
| After 50 days | / | / | 10 S. aureus | 10 S. aureus | |
The number of other tested microorganisms was significantly reduced. Results of the challenge test of the investigated formulations after 28 days, demonstrated a reduction of the number of all inoculated test microorganisms to a minimum by the action of both tested preservatives. After 50 days of storage, only the presence of S. aureus in formulations with potassium sorbate and A. brasiliensis in formulations with both preservatives was determined in very small numbers. Similar results were obtained in creams inoculated with a mixture of all tested microorganisms, where the total number of inoculated microorganisms was completely eliminated or reduced to a minimum with both tested preservatives.
Based on the obtained results, it can be concluded that both preservatives significantly reduced the number of inoculated microorganisms, indicating that they can be used as effective preservatives in cosmetic products with herbal extracts, such as Day and Night cream with marigold extract.
4. CONCLUSION
Preservatives are substances with a crucial influence on the microbiological stability of cosmetic products. This study highlighted the influence of paraben-based preservative blend (Gujsol-1®) and potassium sorbate, on the stability of creams containing marigold flowers propylene glycol extract. All tested formulations showed favorable texture and sensory characteristics, desirable pH values, satisfactory physical stability and appropriate microbiological quality at the initial point, as well as during the entire period of storage of two years. On the basis of these results, it can be concluded that potassium sorbate could be an efficient alternative to paraben-based preservatives, such as Gujsol-1®, in formulations containing plant extracts.